The grapes and apples are picked, crushed and pressed. Everything is a go for fermentation. Except it’s too hot! You want a cool fermentation, around 60 °F (16 °C), but the coolest space in the house is 70 °F (21 °C). Short of buying a new refrigerator or maxing out the air conditioner, what is one to do?
You might consider evaporative cooling, which is a simple and inexpensive way to cool a small fermenter. Many wine and beer styles require cool fermentations (for example, white wines, ciders and lagers). Cool fermentations produce fresher and more fruity wines, helped, in part, by the greater production of aromatic fruit esters, such as isoamyl, isobutyl and hexyl acetates (see R.S. Jackson, Wine Science, 1994). Failure to ferment under optimal temperatures can result in off-styles, or worse, off-flavors. For example, high temperatures (greater than 86 °F/30 °C) can inhibit yeast growth, leading to stuck fermentations and overly-sweet wines.
Evaporative cooling means using the power of water evaporation to cool a fermentation. If the wet evaporating surface surrounds your fermenter, evaporation literally sucks energy, in the form of heat, out of the fermenter. This causing cooling under most, but not all, environmental conditions. Evaporative cooling depends principally on three quantities: wind speed, temperature, and, most importantly, relative humidity.
Relative humidity is routinely measured based on the principle of evaporative cooling using an instrument called a sling psychrometer. This device has two thermometers: a dry bulb, and a wet bulb that has a wick around the bulb connected to a reservoir of water. This device is slung around overhead, causing an evaporative cooling of the wet bulb. With the dry and wet bulb temperatures, one can look up the relative humidity in a table (for example, in the “Handbook of Chemistry and Physics”).
This tabular data can be reconfigured in a way that is more useful for estimating “potential” evaporative cooling for fermentations. The chart below shows the evaporative cooling (dry – wet bulb temperatures) as a function of temperature (measured by the dry bulb) and relative humidity.
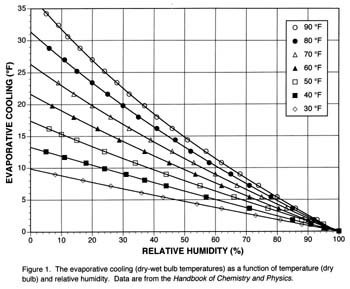
Note that at all temperatures, evaporative cooling goes to zero at 100% relative humidity. If the air is saturated with water vapor, no cooling is possible. As temperature increases or relative humidity decreases, evaporative cooling increases.
For example, at 70 °F (21 °C), the potential evaporative coolings at 20, 50 and 80% relative humidities are 20, 12, and 4 °F (11, 7, and 2 °C), respectively. The usefulness of Figure 1 for fermentation science depends on how much of this potential evaporative cooling can be captured in the real world (see “Experimental Validation” at the end of this article).
Four steps are involved in estimating whether evaporative cooling will work for a specific case. First, what is the desired fermentation temperature? Let’s call this quantity “Wdesired” (e.g., 60 °F). Second, estimate the potential evaporative cooling [ΔT = dry bulb(D) – wet bulb(Wpotential)] from Figure 1. For this step, you will need a thermometer and a relative humidity sensor; these two instruments are often combined and can be bought inexpensively at most hardware or home-improvement stores.
Let’s assume the room temperature is 70 °F (D) and the relative humidity is 50%. Interpolating in Figure 1 gives a potential cooling of 12 °F. Third, calculate the Wpotential = (D – ΔT), which is 70 °F – 12 °F = 58 °F. Fourth, compare Wdesired (60 °F) to Wpotential (58 °F). In this case, evaporative cooling could be used to get near the desired temperature.
If the potential cooling is far removed from the desired cooling, try manipulating wind speed, temperature, and relative humidity (by moving the fermenters around in the house, garage or outdoors, and by using fans) to get a more optimal fermentation temperature.
In our everyday use of this technique, we rely on wicking of water upward from a reservoir to keep our 5-gallon carboys wet. The carboys are completely wrapped in cotton T-shirts and towels and placed in 5-6 inches of water. Cotton is a good water wicker and when wet it clings to the surface of the carboy, which facilitates heat exchange from the carboy.
To prime the wicking process and occasionally to assure a complete wetting of the fermenter, we pour water over the top of the carboys. Glass and stainless steel are good heat conductors, so they would work better for evaporative cooling than plastic fermenters or wood barrels.
Commercial applications of evaporative cooling rely on spraying water from above; these commercial systems can maintain precise temperature control by timing the spraying to the temperature in the fermenters (see pages 168-170 in E. Peynaud, Knowing and Making Wine, 1984). Good ventilation, the efficacy of stainless steel as a good heat conductor, and covering tanks with tarpaulins for better wetting are also methods mentioned by Peynaud. A key to cooling is to keep the outside of the fermentation chamber wet, whether it’s a 5-gallon carboy or a 1,000-gallon stainless-steel tank.
Compared to more high-tech options such as refrigeration, evaporative cooling has advantages, such as low cost, a substantial cooling effect under appropriate environmental conditions (see chart) and scalability. For example, evaporative cooling works for fermenters ranging in size (scale) from 1-gallon glass containers to commercial applications (see previous paragraph).
However, bear in mind that the rate of heat loss from a fermenter is proportional to the surface-area-to-volume ratio. Therefore, small fermenters with high ratios are more effective than large fermenters in dissipating heat. If you anticipate a hot fermentation, start the evaporative cooling early; do not wait until the fermentation temperature is out of control.
Disadvantages of evaporative cooling include lack of precise temperature control in most home environments and little cooling under high humidities (Figure 1). The controllability of a fermentation temperature using evaporative cooling depends significantly on the daily variability in the ambient temperature and the mass of fermenting juice, which serves to buffer temperature change. For example, in our experiments (see sidebar), room temperatures varied by 10° F, but fermenter temperatures only varied by 5° F. The fermenter temperatures always tracked room temperature, but were always offset to a lower, more buffered, temperature. For example, the “wet+fan” treatment averaged 55° F and varied between 52 and 57° F.
In the final analysis, controlling temperature depends on understanding basic principles such as those that control evaporative cooling. So the next time you need to cool a wine, cider or beer fermentation, you might try evaporative cooling, an inexpensive technique that works over a broad range of temperatures and relative humidities. Happy fermentations!
Experimental Validation
We set up an experiment to assess how much of this potential evaporative cooling we could capture in simple home fermentations. To begin, three 5-gallon glass carboys were filled with water and wrapped in cotton T-shirts and towels.
One glass carboy was left “as is” in a shaded room (this was the “dry” treatment, analogous to the dry bulb of a sling psychrometer). Meanwhile, the other two carboys were placed in separate shallow pans of water (approximately 5 inches of water), one without a fan (the “wet” treatment, analogous to a still-air wet bulb of a sling psychrometer) and one with a floor fan blowing on the glass fermenter at a low setting (this was the “wet+fan” treatment, analogous to a wind-aided wet bulb of a sling psychrometer). This wetting technique relies on the wicking of water upwards onto the fermenter from a reservoir.
We measured the temperature in the carboys twice daily. In addition, we recorded the room temperature and the relative humidity; these two measurements were used to estimate potential evaporative cooling by interpolating in the chart.
After a couple of days to acclimate, the experimental treatments reached steady-states. Over the last seven days of the experiment, the mean (± 1 standard deviation) evaporative coolings (?T) for the dry – wet treatments, the dry – (wet+fan) treatments and the potential cooling [Figure 1, Y axis] were 7.9±0.6 °F, 13.2±1.0 °F, and 12.7±0.7 °F, respectively.
Given the experimental variability, there is no significant difference between the potential cooling and the dry – (wet+fan) treatments, which means that the simple treatment of a fan blowing on water wicking up from a reservoir was sufficient to capture 100% of the potential cooling. Even the simpler treatment of water wicking up the carboy in a still-air environment was able to capture 62% of the potential cooling.
The dry, wet, and “wet+fan” treatments averaged 68, 60, and 55 °F over the last seven days of the experiment. These are substantial temperature depressions. The results of our experiment indicate that the chart is a useful guide to estimate the potential for evaporation cooling in the real world.






