Guide to Cleaning and Sanitizing Winemaking Equipment

We can probably all agree that readying equipment for winemaking is not exactly a carboy of fun; nevertheless, it is an absolutely crucial aspect of winemaking. Reports of wine gone bad and, sadly, accidents resulting from improper washing and sanitizing of equipment and handling of chemicals are all too common — pitted stainless steel, expensive oak barrels relegated to planter duty, shattered carboys, and trips to the hospital.
So, what is the difference between washing and sanitizing? Which agents should be used to wash or sanitize a particular material? Does technique matter? How safe are these chemicals? Precise answers to these questions are complicated.
Here, we provide basic information by reviewing the types of chemicals found in washing and sanitizing products, their effectiveness, resistance of equipment to these products, and suggestions for their safe and effective usage.
Understanding Washing and Sanitizing
Washing is the process of removing organic and inorganic contaminants to a level of insignificance. Washing agents are designed to facilitate this process by breaking down, solubilizing and dispersing contaminants into water, so the contaminants can be washed away.
Latent microorganisms are capable of collecting nutrients and defending against attack by sanitizing agents to go on and spoil wine in the making. For this reason, it is important that the washing process be effective and adequately applied.
Winemakers often use the terms “sanitization” and “sterilization” interchangeably; however, they are not equivalent. Sterilization is the process of eradicating all living microorganisms by using specialized technology and methodologies, such as autoclaving. Autoclaving involves exposing items to high-pressure, saturated steam at 250 °F (120 °C ) for 15 to 20 minutes — certainly not a practical option for home winemaking, and it is unnecessary. Pouring boiling water on, or into, equipment is not the same as autoclaving and doing so will not sterilize, it is unlikely to be as effective as using chemical sanitizing agents, and can damage equipment. Sanitization is a limited form of sterilization; it is the process of eradicating living microorganisms down to an acceptable level and which has no adverse effects on winemaking.
Bear in mind that a given washing or sanitizing agent may not be compatible with every type of material in winemaking equipment. An agent that is suitable for use with stainless steel, for example, may severely damage glass, PET (polyethylene terephthalate), oak, food-grade plastics and rubbers, and other types of materials, or vice versa. Washing and sanitizing agents often do not come with sufficient and useful information to help us make informed decisions about which to use with what materials and under what conditions. The information on an agent’s material safety data sheet (MSDS) is intended primarily as a warning regarding personal safety, not equipment safety, and is only required to list non-proprietary chemicals that are considered hazardous and that exceed 1% concentration.
Let’s look at the basics of washing and sanitizing in the home winery, from pre-rinsing to the final rinse, and then review the types of agents and products available to accomplish these tasks.
Pre-Rinsing
As soon as you finish using a piece of equipment and while any organic material remains wet, rinse the equipment with a jet of cold tap water to dislodge the bulk of the material and finish with a jet of hot tap water. The hot water should not exceed 125 °F (52 °C). Water above this temperature is dangerously hot and is not necessary for effective pre-rinsing, and excessively hot water can damage many types of plastic equipment and is likely to cause soft (soda lime) glass carboys to expand unevenly and crack. Keep rinsed equipment wet until you wash it and start washing it promptly.
Preparing Washing and Sanitizing Agents
Soaps are not up to the task of washing equipment and very likely to leave residues (soap scum). Select washing and sanitizing agents that are compatible with your equipment (see compatibility table below) and follow these rules:
- Use hot water that is not more than 125 °F (52 °C).
- Do not use higher concentrations than recommended by the manufacturers of the agents or your equipment.
- Make up your working solutions of agents in a container that is made of a material known to be especially resistant to attack by the agents. Always fill the container with water before adding the agent and when adding the concentrated agent, stir the water vigorously to ensure that the agent is quickly diluted and uniformly mixed. Adding a concentrated liquid or powdered cleaning or sanitizing agent to a container before adding water can damage the container.
Washing
How you wash will depend very much on what you are washing; however, certain generalizations can be made:
- Don’t soak your equipment for long periods in solutions of chemicals that are known to attack the materials from which the equipment is made. (Specifics are given later in the article.) Short exposure may be acceptable, even recommended, but avoid prolonged (overnight or longer) soaking.
- Agitation and cautious rubbing will greatly facilitate the washing process. However, whatever you use for rubbing should be sufficiently non-abrasive so that it will not scratch the surface of your equipment. Scratches will just make the equipment more difficult to wash the next time and can harbor microorganisms and other contaminants.
- It is better to use your working solution of washing agent in modest, repeated doses, rather than all in one shot. The washing power of fresh working solution will be stronger and more effective than a solution that is significantly contaminated with the dissolved and suspended materials you want to wash away.
Easily accessible surfaces are generally easy to wash. Inaccessible or difficult to access surfaces are the real challenge; pumps, hoses and tubing can be especially difficult to wash. Pumps with heads that can be easily disassembled are much easier to wash. And it is much easier to tell if the inside of flexible tubing is degraded or dirty when the tubing is made of transparent or translucent material; however, be aware that not all such tubing is rated for contact with food and some types may scalp flavors, i.e. move flavors/odors between juice/wine and packaging or equipment.
There is a very effective, low-effort way to wash carboys. Combining soaking with agitation is considerably more effective than soaking alone. First add about ¾ gallon (3 liters) of hot washing solution and close the carboy with a stopper that has an easily removable vent plug. Then, place the carboy sideways on a soft surface, such as an old piece of carpet, and slosh/roll back and forth. Stop every few minutes to release the suction that results as the warm water vapor cools and condenses. This simple approach will quickly loosen most of the debris and residues, such as dried lees, except for a residue line around what was the foamy top of the fermenting wine.
If the washing solution gets very contaminated and is losing its effectiveness, dump it out and repeat the process. If some residues do not loosen, put a 12-in. (30-cm) square piece of an old bath towel in the carboy with about a ¼ gallon (1 L) of hot washing solution and repeat the slosh/roll to cause the piece of towel to rub on the residues. Next, add about a gallon (4 L) of washing solution so that when the carboy is inverted (neck down), the line of residues near the shoulder is just submerged, bung the carboy, and invert it in a plastic pail and let the line of residues soak. Remember to release the suction until the washing solution cools to the point that suction is not longer developing. How long you let the carboy soak, before using more agitation, will depend on the nature of your washing agent. If you are using chemicals that can damage your carboy, you will want to check the residue line every hour or two. If you are using a neutral pH enzyme-based detergent, you can let the carboy soak overnight, or longer.
Before moving on to sanitizing, ensure that all surfaces are thoroughly washed and rinsed. Remember: sanitizing agents are not likely to sanitize dirty surfaces effectively. And never mix a washing agent with a sanitizing agent to speed the process — it does not work and may damage equipment, and can be hazardous to your health.
Sanitizing
Sanitize with a suitable sanitizing agent, properly diluted and compatible with the type of equipment being washed. Chemical, as opposed to biochemical (enzyme-based), sanitizing agents typically consist of very aggressive oxidizing agents that should effectively sanitize clean surfaces in less than ten minutes. Soaking equipment in such sanitizers for long periods of time is generally unnecessary and can damage equipment. The effectiveness of enzyme mixtures as sanitizers has not been extensively tested for home winemaking, but may prove to be effective and more gentle on equipment.
Final Rinse
Some non-toxic sanitizing agents (see later) do not require a water rinse; however, a final water rinse is always a good idea. If your municipal water contains too much chlorine, an inexpensive carbon water filter can be used to remove the chlorine, but bear in mind that once the chlorine has been removed, there is nothing to prevent microorganisms from proliferating downstream from the carbon filter. Keep that part of your plumbing short and clean.
After the final rinse, drain your equipment thoroughly and ensure that it is free of odors. If an odor lingers, you might try filling the carboy, or similar equipment, with cold, odor-free water to displace all the air, then drain it and check again for odors. If they persist, cleaning should be repeated.
Washing Agents
Washing products can contain several “active” ingredients, including oxidizing agents such as hydrogen peroxide, acids and bases (alkaline chemicals), surfactants that break down dirt and greasy residues, and chelating agents. Surfactants (short for surface-active agent) are hydrophilic-hydrophobic compounds capable of lifting and dispersing dirt by lowering the surface tension of a liquid, allowing easier spreading, and lowering of the tension between two liquids, or between a liquid and a solid.
Detergents, foaming agents, and wetting agents are examples of surfactants. Chelating agents, such as phosphates, are chemicals that sequester minerals — the culprits in hard water.
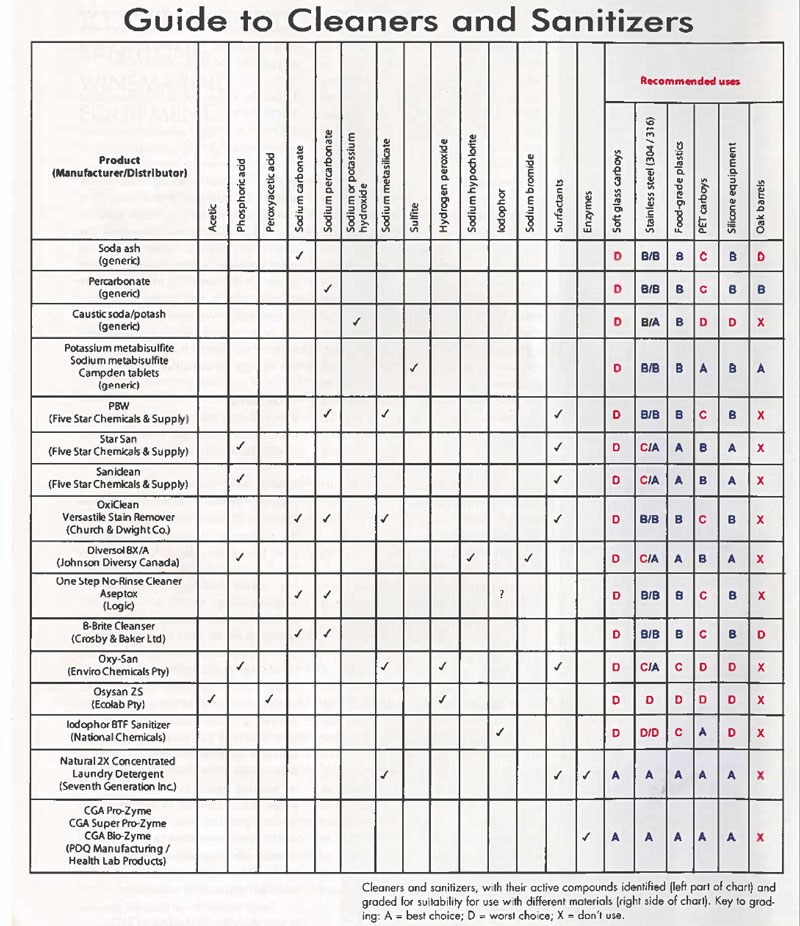
Sodium carbonate and sodium percarbonate are the most common alkaline chemicals found in cleaning products, being very effective in dislodging heavy deposits from tank walls or removing greasy residues. These products often incorporate surfactants and/or sodium metasilicate, an effective flocculant used in wastewater treatment which also inhibits corrosion. A dilute 1% solution of either chemical is sufficient for most applications. Sodium percarbonate has the advantage of dissolving tartrates and neutralizing acetic acid in problem barrels; sodium carbonate is not recommended for barrels as it excessively leaches key oak compounds.
Sodium carbonate does not dissolve easily; so first dissolve the powder in warm — not hot — water and then dilute the solution to the required concentration by adding the proper amount of cool water. Use the solution to wash equipment, leaving the solution in contact with the surface for at least 10 minutes.
Sodium percarbonate is commonly found in household laundry detergents. In water, it dissolves to release sodium carbonate and hydrogen peroxide, a very effective disinfectant and bleaching agent on most materials. It is highly recommended for cleaning oak barrels and for removing excess tannins, and particularly in treating barrels affected by spoilage microorganisms. Store sodium percarbonate solutions in a properly stoppered glass container as hydrogen peroxide tends to break down quickly.
Examples of products that contain sodium carbonate and/or sodium percarbonate include PBW (Powdered Brewery Wash), One Step No-Rinse Cleaner (known as Aseptox in Canada), B-Brite Cleanser and OxiClean, a popular household detergent used by home winemakers and homebrewers.
Caustic alkaline chemicals, notably sodium hydroxide (caustic soda) and potassium hydroxide (caustic potash), are very effective for washing tanks. For most stains, a 1% solution with a short-soak period of 15 minutes followed by a light scrubbing will work well; increase the concentration up to 5% for tough stains. But be careful as these chemicals are very corrosive and detrimental to many other metals and materials, especially glass. They are also extremely hazardous to humans; they can cause permanent skin burns or cause blindness.
In all cases, a thorough rinse with a 1% citric acid solution is recommended to neutralize any alkaline residues.
Sanitizing Agents
Sanitizing products incorporate a range of chemicals that inhibit or kill contaminating microorganisms.
Potassium and sodium metabisulfite, more commonly referred to as sulfite or KMS in the case of the potassium powder version, are the most widely used and most effective sanitizing agents for winemaking applications.
Though sulfite powder dissolves much more readily in hot water, this is not recommended as it reduces the effectiveness of sulfur dioxide. Use a 10% sulfite solution by allowing the solution to be in contact with the equipment for approximately 10 minutes, no longer on stainless steel and silicone bungs as it can cause pitting and cracking with extended use. You can increase the sulfite solution’s sanitizing effectiveness by adding equal parts of citric acid. The solution can be reused for several weeks if stored in a properly stoppered container.
If you have access to fresh, clean water, rinse the equipment thoroughly; otherwise, if you will not be rinsing, use a dilute 1% sulfite solution — any residual amount of sulfite will not have any adverse impact on your winemaking.
Acid sanitizers, such as phosphoric acid, are also effective as they are able to shut down cell membranes in microorganisms. They are used extensively in the food and beverage industry because they leave equipment in an acidic condition that eliminates water spotting.
Star San and Saniclean are examples of acid sanitizers that use phosphoric acid and incorporate surfactants, and are therefore often recommended as one-step cleaner/sanitizers. Oxy-San is another similar product, which also incorporates hydrogen peroxide, sodium metasilicate and surfactants.
The main disadvantages of acid sanitizers are that they are corrosive to soft metals and may kill selected microorganisms, potentially leaving behind latent microorganisms that can then strike at an opportune time to cause spoilage.
Peroxyacetic acid (PAA), found in such products as Oxysan ZS — where it is used in conjunction with hydrogen peroxide — is an excellent bleaching alternative to chlorine and chlorine dioxide sanitizers. PAA is considered eco-friendly because it breaks down into acetic acid and hydrogen peroxide with minimal foaming, which do not require rinsing; and it is recommended where soft water is not available. PAA acts very quickly, but it has a pungent odor and is very expensive, relative to other sanitizers.
Bleach (chlorine) solutions have long been used for sanitizing equipment and specifically for removing stubborn stains on glass carboys, for disinfecting, and for decolorizing equipment when switching from red to white winemaking; it is also used in wastewater treatment.
These are generally more effective than acid sanitizers and the cheapest of sanitizers but probably the least environmentally friendly.
Dilute bleach solutions are prepared from sodium hypochlorite and/or chlorinated trisodium phosphate (TSP). TSP is a stronger and therefore more effective alkaline chemical than sodium carbonate, and is a common component of household laundry products.
Diversol BX/A is a very common chlorine-based product used by home winemakers.
Exercise great caution with these products. Some are irritants and very strong oxidizers and highly corrosive and should therefore not be used on stainless steel; pitting will result, particularly in scratched areas. They are also not recommended on plastic equipment and in oak barrels as the material will absorb chlorine, making it difficult to remove and likely cause spoilage. And never mix acid or ammonia with bleach to try and improve effectiveness; the result can cause the release of toxic chlorine gas. And products containing ammonia should never be used because they can become a source of nitrogen nutrients for microorganisms in the winery or wine.
The major drawback of chlorinated agents is that it they can be a source of the nasty chemical 2,4,6-trichloroanisole, better known as TCA, the compound responsible for the moldy, musty smell in so-called “corked” wines. If you have to deal with stubborn stains on glass and want to use chlorine bleach, do so outdoors well away from any winemaking equipment or area. Powder particles can easily become airborne, find their way into your home winery, and contaminate equipment, which could then spread out of control throughout the whole area. Trying to eradicate a TCA infection could prove to be a formidable challenge, if not impossible.
Use a 0.1% bleach solution, fill containers that need to be sanitized and let stand for at least 10–15 minutes. Do not let sit overnight in glass carboys (or any containers in general) as this may adversely affect the integrity of the material. Thoroughly rinse the containers at least three times with plenty of water as chlorine is harder to rinse and can leave behind a nasty smell. Then rinse the containers with a sulfur-citric solution followed by a thorough water rinse. There should be absolutely no trace of chlorine solution or smell after the water rinse; otherwise, wine will inherit an off-odor and/or off-flavor.
Chlorine dioxide is a more powerful oxidizer and sanitizer than chlorine; it is used for water treatment as an environmentally safer alternative. But chlorine dioxide can release toxic chlorine gas and requires special handling; its use is not recommended in home winemaking applications.
Iodophors are sanitizing agents and surfactants containing iodine, which is similar to chlorine in killing microorganisms though much safer. One such common product is Iodophor BTF Sanitizer. Iodine has low toxicity and easily attaches to organic matter to provide good sanitizing action. Though not as effective as other sanitizing agents, its major advantage is that it evaporates directly from solution to gas when used in proper proportions, and hence leaves no residues and, as some manufacturers suggest, requires no rinsing; however, they tend to produce a lot of foam and can leave an odor, and therefore do in fact require quite a bit of rinsing. Iodophors are generally not recommended on plastic equipment as it can leave unattractive orange-brown stains. Iodophor solutions also stain hands.
Quaternary ammonium salts, or QUATS, are ammonium-based sanitizing agents also used as surfactants as well as disinfectants and fabric softeners in other non-winemaking applications. Their antimicrobial activity stems from their ability to enter cell membranes of microorganisms and disrupt key cell functions. QUATS are most effective at the boiling temperature of water, or 212 °F (100 °C), but are not recommended for use in hard water.
Their greatest advantages are that they leave no odors that might impact wine quality, have very low toxicity, and are not corrosive. Their drawbacks are that they become deactivated by soaps and some detergents, may have reduced effectiveness in the presence of organic matter, are relatively high foaming, and can be a source of nitrogen nutrients to microorganisms.
Safety
Cleaners and sanitizers can potentially be hazardous. Always read the instructions and use them safely.
Never mix chemicals unless your instructions tell you to do so. Mixing chemicals can, in some cases (such as mixing ammonia with bleach), release toxic fumes.
Only use chemicals in a well-ventilated area. If needed, wear gloves, aprons and safety goggles. (Generally, gloves are all you need with most winemaking cleaners or sanitizers, but more protection should be worn when using strong chemicals such as caustics.)
The Material Safety Data Sheet (MSDS) for each product will supply information about its safe use, including first aid. Cleaning winery equipment is not a carboy of fun, but a clean carboy can hold a lot of amazing wine.
Guide to Cleaners and Sanitizers
Cleaners and sanitizers, with their active compounds identified (left part of chart) and graded for suitability for use with different materials (right side of chart). Key to grading: A = best choice; D = worst choice; X = don’t use.