Q I was careless, and let air get into a tank containing about 60 gallons (227 L) of Cabernet Sauvignon. It now has a slightly stale oxidized taste. Is there a way to recover the wine?
John Bolles
Simi Valley, California
A Don’t worry, it’s happened to the best of us! If you can, check your pH and your VA (volatile acidity) to try to get a handle on whether or not this air intrusion caused any chemistry-altering damage to the wine. What I mean is, sometimes when we have an oxygen incursion it can mean an increase in spoilage bacteria that can lead to high VAs, film yeasts, and other issues. For this reason, if you have the capability, I’d sterile filter this wine immediately to rule out the possibility of a high count of spoilage organisms in the wine. That being done, you can focus on some of the following approaches to try to “recover” the wine and nudge it back to where it was heading before it got a big oxygen shock.

Get it topped, adjust pH (if needed), and add SO2: If you can filter your wine, do it as soon as you can to remove the possibility of microbial damage. Then, adjust the free SO2 up to a healthy zone, which for a filtered red wine like Cabernet can often be 25–35 ppm, as long as your pH is under control. What I mean by under control is subjective. I’ve given up chasing the holy grail “molecular SO2” of 0.8 ppm. Molecular SO2 is a measurement based on both the pH and free SO2 of a wine, and to achieve 0.8 ppm, more often than not your pH has to be very low, your SO2 level very high, or both. Because of style and taste (hey, we want our wines to taste good, right?), getting any wine, especially a red wine, to conform to those parameters is just not realistic. For that reason, I choose to have my pHs for reds rest between 3.5–3.75 and my free SO2 between 25–35 ppm. I’ve found over the years that that’s a pretty good sweet spot for me, as long as I’m topping barrels monthly and monitoring my oxygen ingress as well as VA levels.
Blend out the defect: Don’t forget that blending is always an option . . . as long as you have other wines. Do your winemaking buddies have some gallons they could lend you this year in exchange for replacement in a future year? In a pinch, and only with full disclosure to your friends (no bragging rights on bought wine!), I do think it’s OK to purchase a bottle or two off of the shelf to be able to blend something down. However, this probably won’t work with a 60-gallon (227-L) lot — that’s a lot of wine you’d have to buy! However, it’s likely that you’ve got other avenues, so read on below.
Sometimes all a wine needs, especially a red like a Cabernet, is a little bit of oak.
Try an oxidation-specific fining agent: Most winemaking supply companies (AEB, Laffort, Scott Labs, etc.) will have a fining agent that specifically targets oxidized aromas and flavors. Often, they contain PVPP, and may strip color, so do be sure you do bench trials first. Have a chat with a sales representative or check out their websites and it’s likely you’ll find something that could help.
Try refreshing with oak chips: Sometimes all a wine needs, especially a red like a Cabernet, is a little bit of oak. If your wine got a big hit of oxygen, I’m guessing it lost some of its fruit character. There are a number of oak chips on the market that can help contribute not exactly fruity flavors, but elements like spice, coffee, or vanilla. These chips don’t actually have spice, coffee, or vanilla in them — they are just specifically sourced and toasted to express those characteristics in wine. For a barrel or even a carboy, you can easily make a little chip “sock” by putting small chips into a mesh or nylon bag. A nylon stocking makes a perfect single-use bag. To punch up some new aromas and add back some of the antioxidants you lost (also, see “Try a fining tannin”, below) experiment with about 1–2 g/L chip addition and see where that takes you. As always, be sure to buy chips from a reputable supplier with high turnover for freshness and best quality.
Try a fining tannin: As logic would have it, you’ve introduced oxygen, which has reacted with some of the natural antioxidants in your Cabernet (the color and tannin molecules). So it would make sense that now you have to build them back up again, right? Some of my favorite, and certainly the most versatile, winemaking aids I’ve discovered in the last five years are the new breed of oak and grape tannins available on the market. The aforementioned winemaking supply companies all carry different kinds of tannins that can be added during fermentation, aging, or even just before bottling, to boost body, grip, or even to give a subtle oak flavor. They are extracts from oak and grape skins, typically, so are just another way of introducing some of the elements you’d get from a barrel or from fermentation. They can also do wonders in reviving and refreshing a wine that is getting tired from age or suffered an untimely accident, like yours. Again, be sure to do bench trials so you see what works and at what levels. All suppliers should be willing to give you a tiny packet or vial with which to do trials before making a purchase, and likewise should be able to guide you towards which of their products they think would work the best. Good luck — I have faith that your wine is not beyond rescue.
Q Because of travel restrictions over the past few months, I was unable to rack my 2019 (New Zealand) Pinotage from a Speidel plastic fermenter to the stainless container or adequately manage sulfite levels (basically I was unable to travel back from the U.S. to New Zealand until recently). I could taste volatile acidity (VA) and sent the wine off for testing:
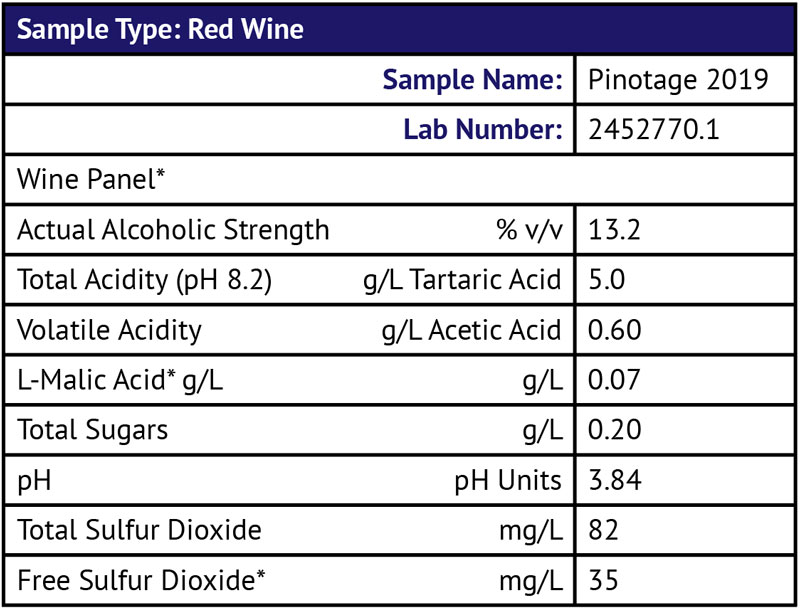
I have read that the legal threshold for VA is 0.7 g/L and blending is the best solution. So far, I have added another 1 g/L of tartaric acid and bumped up the sulfite to 75 ppm. Unfortunately, I don’t have any blending wine. It is OK to drink, especially with food, so I am reluctant to dump it. Do you have any suggestions? I don’t normally filter my wines — should I pass through a sterile filter before bottling, any other way to reduce the VA or minimize the sensory impact and can I prevent it deteriorating further?
Mary Rogan
Auckland, New Zealand
A Sadly, blending VA levels downward remains the only option available for reducing VA content in small lots. Larger commercial wineries, with big lots and bigger pocketbooks, can afford the expense of reverse osmosis and distillation technology to remove it from their wines but there’s no affordable option for small producers. Simple filtration doesn’t work to remove VA, though sterile filtration can certainly remove the spoilage yeast and bacteria that may be contributing to it.
The good news for you is that your VA levels are nowhere near approaching legal limits. The legal limit for VA in the U.S. is actually 1.1 g/L for white wines, and 1.2 g/L for red wines. At these levels, I indeed do find the wine objectionable. Sensorially, at 0.6 g/L, you should be within a tolerable range, especially for a wine that is already a year old. VA tends to climb with time and even though you had a moment during the wine’s lifetime where you weren’t able to keep the SO2 levels up in ideal ranges, it’s actually not that bad. It is possible, however, that you’re also smelling some aldehydes or acetaldehyde, which can smell like nail polish remover or paint thinners. If that’s the case, your SO2 addition should help bind up some of these and reduce the perception of those offensive aromas.
At a free SO2 of 35 ppm and a total SO2 of 82 ppm, I wouldn’t worry about the wine being safe to consume; it certainly is. Many wines are bottled with free SO2 in that range and though I’d certainly be wary of big additions in the future, your big add should tide you over for at least a couple of months as long as the wine isn’t exposed to oxygen and isn’t carrying a high microbial load. If you do suspect you’ve got spoilage bacteria (if you have any film yeasts growing, or if the wine is cloudy and spritzy where it should be clear and settled), you might want to filter it anyways.
I’m glad that you added some tartaric acid to get your pH down — that’ll help prevent some of the inevitable future VA creep. (For other reader’s reference, it always helps me diagnose a problem when I can see a wine’s analysis; it gives a bigger picture, so thank you very much for including yours!) High pHs, especially coupled with oxygen exposure and low SO2, are notorious for leading you down the path to VA issues. Indeed, VA prevention is something about which an entire book can be written. Just give a search on our homepage, winemakermag.com, for some great free articles and member’s-only content. VA is definitely something where an ounce of prevention is worth a pound of cure.
For your wine, in your case, I’d say that if you like how the wine tastes, feel free to keep it as topped as possible, with your free SO2 levels up between 25–30 ppm, and see how it ages over the next month. I know it sounds a little blasphemous but, in the past, I have told readers (if they don’t feel like it’s breaking all the rules) that it’s OK to buy some wine to blend if it helps rescue a batch. Just be sure to tell your friends and family full disclosure that it’s not all yours . . . if it turns out tasty!
Q My red wine measures tartaric acid: 50%, malic acid: 25%, and lactic acid: 25% by chromatic paper. At what % lactic acid is it safe to add sorbate so wine can be sweetened a little and not risk negative results. Can you add a reduced amount of sorbate if necessary?
Rich Romanowski
Greenwood Springs, Mississippi
A Winemakers typically add sorbate (aka sorbic acid, often purchased as potassium sorbate) when they want to bottle a wine with a little residual sugar. It is often added right before backsweetening and bottling. Sorbate will inhibit the reproduction of yeast cells but it will not “kill” yeast, nor will it inhibit or kill bacteria. It should always be added in the recommended doses; too short of a dose will not inhibit the yeast from continued growth.
As you hint in your letter, if you add sorbate before the ML fermentation is complete, you run the risk of a geranium-like off-odor that can really ruin the aromas and flavors of your wine. For this reason, I recommend that you wait until your “malic acid spot” on your chromatograph disappears entirely, which means that you’ll have an ML complete wine.
It’s really difficult to determine the amount of lactic acid you have based on the size of a spot on a chromatograph, so I always have just gone with the “malic spot” disappearing. Because chromatography is tricky to do and somewhat inexact, I’ve always been a fan of sending in my wine for malate analysis when you think it might be done (often when you hear the little CO2 bubbles stop ‘ticking’ in the fermenter).
Q Recently I conducted a paper chromatography test to check the status of malolactic fermentation introduced this fall into a number of 5-gallon (19-L) Marquette carboys produced from my backyard vineyard. Results showed plenty of malic acid still present. I plan to cold stabilize over the winter months in my winery. Will malolactic fermentation under these circumstances naturally kick back in when temperatures rise in the winery in the spring? Or is it best to re-inoculate with new culture and nutrients in the spring?
Jim Boutin
Pownal, Vermont
A I always think it’s wonderful when people can do a “natural” cold stabilization over the winter months. It’s an incredibly intuitive and very old-fashioned, non-interventionist way to accomplish a key winemaking task. It never gets cold enough here in Napa, California during the winter to really knock down any significant amount of our tartrate crystals (the goal of cold stabilizing a wine), so that’s very cool (LOL) if you can do it. I do indeed think that it’s very likely that your malolactic fermentation will re-start once the weather warms up.
Instead of re-inoculating (malolactic bacteria are expensive!) you could wait and see if you start to get any activity when the temperatures start to rise. You can do this by monitoring the disappearance of the malic acid spot on your paper chromatography test. In addition, you can also listen at the mouth of your carboys for the little “tick . . . tick . . . tick” of carbon dioxide bubbles being produced by an active malolactic fermentation.
To be sure that you’re detecting any change, do a chromatography measurement right before you put your wines out in the cold to start stabilizing. On your paper, since the spots will fade over time as the solvent degrades, very carefully (don’t touch the paper with your hands, use gloves as the solvent is toxic) draw a pencil line around the little malic blob. This way when you test again in the springtime months, or whenever you believe malolactic fermentation has resumed, you’ll be able to see if the size of your spot has shrunk and if activity has indeed kicked back into gear.
If you don’t see any signs of activity after the weather has been warm (above 55 °F/13 °C) for a few weeks, and if you’ve tried things like bringing your carboys inside or wrapped them with electric blankets, then it’s time to consider re-inoculating. Make sure that the alcohol isn’t too high (>14.5% is inhibitory), the pH too low (<3.3 is inhibitory), or the temperature too low (>55 °F/13 °C is ideal).
I’m so glad that you mention malolactic nutrients! So many people just toss in a bunch of bacteria and hope for the best, not realizing that malolactic bacteria are even more fastidious in their nutritional needs than yeast cells are. Especially since the wine has been sitting for a while, it’s possible that many things have fallen to the bottom of the vessel or have been consumed (albeit slowly) over the winter’s nap and are now unavailable. Re-supplementing with a nutrient at this time is key.
Additionally, be sure not to add any SO2 as malolactic bacteria are extremely sensitive to sulfur dioxide. Hold that addition until you have confirmed completion. Good luck with your cold stability, and I hope that your wine does reignite its malolactic fermentation in the spring. If not, re-inoculating is nothing to be ashamed about!







