The Relationship of pH and Acid in Winemaking
Home winemakers know pH and acid are related when they make wine. Beyond that, the details sometimes get a little fuzzy. Shedding some light on how these important parameters are — and are not — linked to one another may help make better winemaking decisions. There are theoretical considerations from organic chemistry and laboratory analysis, practical effects on the wine, and a range of steps that can be taken if a change in pH or acid is desired.
Of the two, I will start with pH. It represents just one thing — the concentration (or “activity”) of free hydrogen ion (H+) in a given solution. “Acid” on the other hand encompasses several different compounds in wine. The pH scale runs from zero at the highly acidic end of the scale to 14 at the highly basic (caustic) end. Pure water has a pH of 7.0, right in the middle. Everything below pH 7 is called acidic and everything above 7 is basic, with 7 referred to as neutral pH. To express the concentration of hydrogen ion on the pH scale, we look at the molar concentration written as [H+]. That value is then converted to scientific notation using an exponent to generate a logarithm. For instance, if [H+] = 0.01 M, that can be expressed as [H+] = 10-2 M. The exponent is -2, so the base-ten logarithm is -2. To avoid working all the time with negative numbers, the convention is to use the negative logarithm of the hydrogen ion concentration, so the sign reverses –(-2) = 2. The lowercase p in front of H in pH means just that: “the negative logarithm of.” And the H represents the molar concentration of the hydrogen ion.
So now we know that a solution with 0.01 M hydrogen ion = 10-2 M and the negative logarithm of that figure is 2: a 0.01 M hydrogen ion activity means pH = 2! There, that was simple, wasn’t it?
If we represent any acid molecule as consisting of at least one hydrogen atom that can be released in water to become H+ and call the rest of the acid molecule A-, or acid anion, then the reaction of dissolving in water can be represented as:
HA ⟺ H+ + A- (for a weak acid)
HA ⟹ H+ + A- (for a strong acid)
The acid on the left breaks apart (or dissociates) in water to release hydrogen ions and characteristic acid anions. For strong acids like hydrochloric acid, the tendency to dissociate is very strong (denoted by ⟹) and the concentration of hydrogen ion (which determines the pH) is essentially identical to the concentration of acid that was dissolved in the water. Not so with wine, however!
The acids in wine are all weak organic acids. They are weak in that they do not dissociate completely (denoted by ⟺) in water, unlike strong acids, and reach equilibrium with the ions. They are organic in the chemical sense — they contain carbon atoms (we aren’t talking about the farmer’s market meaning of “organic” here). Beyond that, the various acids in wine or must are considered in groups depending on their effects on the wine. The most important group, and the one under primary consideration here, is called the “fixed” acids. These are non-volatile acids that either arise naturally in the grape as it matures or are developed in the wine during fermentation. The volatile acids, on the other hand, with acetic acid being the major one, arise from yeast fermentation, metabolic activity by lactic acid bacteria (as during malolactic fermentation), from acetic acid bacteria (including from rot-affected grapes), or from chemical oxidation of acetaldehyde. They are called volatile because they can be steam-distilled out of the wine for analysis. The fixed acids cannot be steam distilled.
The major fixed acids from grapes are tartaric acid and malic acid. There may also be lesser amounts of citric acid. The fixed acids from fermentation include lactic acid (the product of malolactic fermentation), succinic acid, and a few other minor acids that need not concern us here.
For this discussion of relationships among pH and the acids, I will concentrate on tartaric, malic, and lactic acids. From that basis, we will jump into the complex considerations of somehow correlating these important wine parameters. It turns out that pH and acid are related in value and related in function, but not interchangeable in either.
The common pH range in wine is from about 2.9 to about 4.0. Much below 2.9 and the must is too acidic to ferment effectively and much above 4.0 and the wine is subject to oxidation and microbial spoilage. Those limits invoke the most important reasons for knowing the pH of a wine. While primary fermentation may proceed as low as pH 2.9, most malolactic bacteria cultures have a viability lower limit of 3.1 or 3.2 pH. Meanwhile, the effectiveness of sulfur dioxide (SO2) is strongly pH dependent. The molecular and bisulfite forms of SO2 are generally considered the active inhibitors of microbial spoilage and oxidation. Red wine is adequately protected by about 0.5 mg/L (ppm) of molecular sulfur dioxide and white wine by about 0.8 ppm. We do not measure molecular sulfur dioxide, however, instead analyzing for free SO2 — mostly bisulfite ion, HSO3- at wine pH. To achieve the protective levels of molecular SO2 in a wine at pH 2.9 requires free SO2 at about 11 ppm for white or 7 ppm for red. If we go up near the high end of wine pH at 3.8, the requirement increases dramatically. A white wine at that pH would need a free level of 79 ppm and a red would need 49 ppm. Those levels border on detectible by most wine drinkers and are above the threshold for sensitive tasters. The wine pH also affects red wine color. Many of the colored anthocyanin polyphenolics that give red wine its hue are either colorless or bluish at higher pH.
Flavor changes with pH as well. Low pH wines are fresh, bright, crisp, and possibly sour. High pH wines are round, soft, fat, and possibly flabby. When it comes to flavor, though, we are venturing into discussing the acids more than just the pH. Maintaining solutions at a constant level of acidity, human tasters generally rank the fixed acids of wine in terms of acidic taste as malic > tartaric > citric > lactic. That means, for a given acidity, malic acid produces the sharpest taste and lactic acid the mildest. At a constant pH in a given wine, the order of taste impact shifts to malic > lactic > citric > tartaric. Acid flavor in wine, then, is made up of a combination of the particular acids that are present, along with the total concentration of those acids. “Total acid,” itself, deserves some consideration in evaluating these relationships. Chemically, “total acid” in wine would represent the sum of all acidic compounds present, including the trace materials we are disregarding here. As a practical matter, winemakers deal instead with Titratable Acidity or TA. Just as the name suggests, that is the total of acid that shows up in a titration test. While it may well include some of the minor acids, their concentrations are so low that TA practically considers tartaric, malic, lactic, citric, succinic, and acetic acids. In the United States, TA is titrated with a standardized solution of sodium hydroxide to an endpoint pH of 8.2 (the phenolphthalein indicator endpoint) and expressed as tartaric acid. That is, the TA figure you get is the result of a titration expressed as though all the acid present was tartaric. Normal levels in wine range from about 0.4 g/100 mL (or percent weight per volume) to about 1.2 g/100 mL. Some references refer to TA in grams per liter instead of percent or g/100 mL. To use the figures interchangeably, just recall that one liter is ten times the volume of 100 mL. So a TA in percent can be represented in g/L as ten times that number. For example, a TA of 0.65 g/100 mL = 6.5 g/L. Because the reporting factor of 10 is so much larger than the range of normal TA’s, there is seldom much confusion. The vast majority of wines have TA values between about 0.45 g/100 mL and 0.7 g/100 mL. So if your TA is reported with a value less than 1.0, it is probably in g/100 mL. If it is greater than 4, you are looking at g/L.
Every acid has a characteristic tendency to dissociate in water — that determines how much hydrogen ion is released and the resulting pH. That figure is the acid’s dissociation constant, Ka. Like pH, acid dissociation constants are usually expressed as the “negative logarithm of” as pKa. In a solution of pH equal to its pKa, an acid is half dissociated—it is the characteristic equilibrium pH for that acid. If an acid has just one releasable hydrogen atom, it has just one pKa. If it has more hydrogens, there is a different (and higher) pKa for each additional hydrogen ion released. For the four acids we are considering here, the figures are shown in Table 1.
At wine pH levels, only the first hydrogen atom — the lowest pKa — of each acid is significantly dissociated. At equal concentrations in water, an acid with a lower pKa will produce a lower pH than one with a higher pKa. The common acids in must and wine are not at equal concentrations and the one with the lowest pKa, tartaric acid, dominates the mixture. At about 2⁄3 of the acid in must, tartaric acid represents about 0.5 g/100 mL in a must with 0.75 g/100 mL TA. Malic acid makes up the bulk of the rest, at about 0.25 g/100 mL. At these concentrations, in a pure distilled water solution, the pKa values should dictate a lower pH than is actually observed in must and wine. In Concepts in Wine Chemistry, Yair Margalit calculates that the theoretical pH of such a solution would be about 2.17 and notes that value is well below the observed pH range of must and wine. In Wine Analysis and Production, Bruce Zoecklein, et al, explain that, “hydrogen ion concentration (pH) and titratable acidity (TA) in mature fruit cannot be explained simply in terms of the concentrations of organic acid anions.”
Both books attribute much of the difference, if not all of it, to the action of the element potassium in grapes. Like hydrogen, potassium ionizes to produce a singly-charged positive ion or cation: K+. In grapevines and grapes, the organic acids are produced in physiological processes that leave them with their associated hydrogen ions, more or less dissociated, depending on the pH. As grapes mature, other processes in the vine exchange potassium ions for hydrogen ions. We can represent the two dominant acids in grapes, tartaric and malic, with a shorthand notation of H2T and H2M. (In this notation, “T” and “M” are standing in for the much more complex chemical formulae of tartrate and malate, the structures of which are not important to this discussion.) As hydrogen is exchanged out for potassium, some of each acid turns into a partial potassium salt: potassium bitartrate or potassium bimalate. These may be represented in a similar format as KHT and KHM. With all of the tartrate and malate still present, but fewer hydrogen ions on hand, the pH comes out higher than would otherwise be predicted. The pKa of some of each acid has been effectively pushed up toward the second value.
The unknown concentration and unpredictable effect of potassium account for one of the most challenging facts about pH and acid in winemaking. The pure prediction of pH from acid content or acid content from pH is confounded, leaving the winemaker needing to test both parameters to characterize must or wine. Although pH dip-sticks exist, they are usually lacking in accuracy for the very narrow range of pH values encountered in wine. A properly calibrated pH meter, reading at least to 0.1 pH and preferably to 0.01 pH, is to be strongly preferred. That preference is due to the relatively large difference that can be hidden by the meter that reads only to 0.1 pH. For instance, a reading of “3.2 pH units” may actually be any value between 3.1 and 3.3, since the accuracy is +/- 0.1 pH unit for any reading. That is a significant potential gap for winemaking.
As noted earlier, TA is defined as a titration with standard sodium hydroxide titrant to a pH of 8.2. Several kits are available for the test, using a visual indicator of the phenolphthalein endpoint. While the kits are adequate for many purposes, better accuracy can be obtained using a pH meter to detect the endpoint at an actual value of 8.2. A class A buret, instead of the syringe typically included in a kit, will also improve accuracy. In Techniques in Home Winemaking, Daniel Pambianchi details the tests, and cautions that sodium hydroxide solution should be used within six to nine months or should be restandardized.
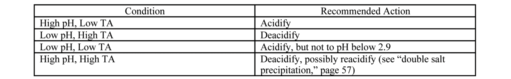
Once the winemaker has the pH and TA results in hand, it is time to consider any needed corrections. In considering “acceptable” values for pH and TA, there are differences based on grape variety, style of wine, local traditions, and viticultural practices. In the main, however, we may note that good white wines will have a pH between 3.0 and 3.4 with a TA between 0.5 and 0.9 g/100 mL. Red wines are usually a bit less tart, with pH between 3.3 and 3.7 and TA between 0.4 and 0.7. When measured values fall into (or even very near) these desirable ranges, go ahead and make the wine without adjustment. If results are outside these ranges, they will usually fall in complementary directions. That is, if pH is high, acid will be low. If pH is low, acid will be high. On rare occasions, a must may have a low pH and a low TA or a high pH and a high TA. In these latter cases, it is potassium that again confounds the results.
Exceptionally low potassium levels produce a pH closer to the theoretical level — lower than normal for wine — while still leaving TA intact. The low/low condition results. Just adding potassium will not help because the ion exchange must take place in the grapevine. At the other end, very high potassium levels exaggerate the usual elevation of pH. This can cause the high/high condition and is difficult to treat.
For problem musts with results outside the ideal range, Table 2 suggests a general course of action with details following.

Acidify
The most straightforward acid addition is tartaric acid. It is the dominant acid of grapes and TA is measured directly in its units. If you have a must with, say, 0.35 g/100 mL of TA, that represents 3.5 g/L. To get to a moderate but acceptable 0.5 g/100 mL (or 5 g/L), you simply add 1.5 g/L tartaric acid. As with most wine adjustments, this should be done as early in the fermentation as possible. However, to avoid over-correcting and the need to adjust back again later, I usually recommend making just one half of a calculated addition, wait a few days, and test again to verify the effect. If still necessary, go ahead and make the other half of the addition. During wine aging, tartaric acid — added or not — may precipitate with potassium as potassium bitartrate. These are the crystals that form during cold stabilization of wine—or form later when an unstable wine is bottled and chilled. Besides the increase in TA, as a rule of thumb, the addition of 1 g/L of tartaric acid will lower the pH by about 0.1 pH units.
Malic acid may be used for acidification, but it is subject to conversion to lactic acid if a malolactic fermentation is carried out. Malic acid will not lower pH because of its higher pKa. Citric acid is not usually recommended for grape wines as it is subject to bacterial conversion to acetic acid.
Deacidify
For most red wines, many Chardonnays, and a few Sauvignon Blancs, the choice for deacidification will be malolactic fermentation. A selected malolactic bacteria strain is added to the wine, usually following primary fermentation. The bacteria convert malic acid, which has four carbons and two active hydrogens, into lactic acid, with three carbons and one active hydrogen. The extra carbon goes off as carbon dioxide. Since the TA titration counts all reactive hydrogens as though they were from tartaric acid, the lactic acid contributes only half as much to TA as the dual-hydrogen malic acid it replaces. With malic acid as 25–35% of the TA, a decrease of about 12–17% of TA may be expected.
In some very high acid musts, the technique of “amelioration” is used. Another name for dilution, this technique adds enough water to lower the TA to acceptable levels. Of course, that lowers the sugar level and all other components as well. Sugar may be added back, but the wine usually suffers from dilution of flavor, aroma, and color compounds. This technique should only be considered for minor adjustments.
Chemical deacidification may be done with calcium carbonate or potassium bicarbonate. The mode of action is slightly different with each of these, but both neutralize only tartaric acid, as it is the most reactive acid in the natural blend. An addition of 0.66 g/L of calcium carbonate will reduce tartaric acid by 1 g/L, but may leave the wine with a bit of an off-flavor. Potassium bicarbonate does not contribute calcium instability. It is effective at about the same use rate as calcium carbonate. Adjustment of more than 2 g/L of TA should be avoided due to risk of bitter flavors from excess potassium ions. Potassium carbonate may be used in place of bicarbonate, requiring 0.92 g/L to decrease TA by 1 g/L.
Double salt precipitation is the most aggressive chemical deacidification. It has the advantage of removing both malic and tartaric acid and is the method of choice for treating extremely high malic acid. If used on a high acid, high pH must, it can be followed with addition of tartaric acid as described in acidification, above, in an effort to get the pH to a lower level. Usually carried out with a commercial product like Acidex, a portion of the wine (usually 35 to 50%) is treated to bring the pH above 4.5 and cause the precipitation of the double salt calcium malate tartrate. The precipitated material is filtered out and the heavily deacidified portion is mixed back in with the remainder of the wine to strike a new pH/TA balance.
With all acid and pH adjustments, up or down, keep in mind the primary effects of these linked parameters. As a winemaker you may ultimately need to make a choice between stability, as represented by pH or taste, as represented by TA. I usually come down on the side of good taste over best stability. If I need to drink that wine young, it may be a sacrifice, but I’m sure I’ll be up to it!





