Yeast Nutrient Strategies
Grape juice is a pretty tough environment if you’re a yeast cell. The pH is low, there’s high osmotic stress (stress from the environmental conditions being such that the flow of water out of the cell is favored), and often, essential nutrients are limited. The abundance of fermentable sugars, however, makes life in these challenging surroundings worthwhile for our single-celled eukaryotic friends.
Our charge as winemakers is to try and ease some of this stress so the yeast can efficiently convert the sugars to ethanol, and hopefully produce some delicious aroma compounds along the way.
One way to alleviate yeast stress is by providing proper nutrients. This article will shed some light on why yeast need nutrients and what they do once they have them. I hope this knowledge will help when making decisions about what nutrients to add and when to add them in the winemaking process.

Before we get too far along, let’s review classes of nutrients required by yeasts for efficient fermentation, so we can talk about each one in more detail below.
Nitrogen – Yeast require nitrogen and yeast assimilable nitrogen (YAN) is the portion of nitrogen in grape must that yeast will utilize. Nitrogen is the most likely nutrient to limit yeast growth during fermentation, which is why it is the main ingredient in most commercial yeast nutrient additives.
Carbohydrates – Sugar is a carbohydrate. This is the precursor for everything the yeast produces, including alcohol. Luckily this is usually present in sufficient quantities.
Oxygen – Fermentation is an anaerobic process (one that occurs without oxygen); however, the yeast do require small amounts of oxygen early on for efficient fermentation.
Vitamins – Just like people, yeast need their vitamins to stay healthy.
Minerals – These are required to maintain osmotic balance (the right level of water within the cell) and also in some reactions.
All of the yeast’s metabolic pathways are interconnected. For example, if there’s plenty of sugar available, but nitrogen is limited, then fermentation might proceed, but the yeast cells won’t be happy. This is because they won’t be able to make proteins or cell walls, and won’t be able to reproduce; this will lead to a low yeast population, causing a slow or stuck fermentation. Because of the inter-connectedness of the pathways, it is important to think about all of the possible (unintended) consequences of nutrient supplementation. Think of it this way: when doctors prescribe treatments, their first responsibility is to do no harm to their patients. And similarly, with wine-making sometimes the best course of action is to not make any additions — the “do no harm” approach. If you know there is an intervention that can help fix a problem, however, or better yet prevent one, then it’s best to act.
Nitrogen
Grape must contains a variety of nitrogenous compounds including ammonia (AMM), amino acids (AA), peptides and proteins, but not all of them can be assimilated by yeast. Primary amino nitrogen (PAN) and ammonia are used by yeast, and are known collectively as yeast assimilable nitrogen (YAN). When Saccharomyces cerevisiae ferments grape juice into wine, it requires considerable amounts of nitrogen to produce yeast biomass. This means that the more assimilable nitrogen present, the more likely yeast cells will reproduce and create more yeast cells; with a larger population of yeast cells, fermentation will be faster and less likely to get stuck. In addition to stuck fermentations, low nitrogen can also trigger the production of volatile sulfur compounds (VSCs), which generally have stinky aromas reminiscent of rotten eggs and cooked cabbage. This is probably not the style you’re going for in your wine! The good news is that even though some VSCs are produced during every fermentation, the carbon dioxide that is also produced helps to “blow off” some of the off-notes. So, while low nitrogen might create a bit of a stink in your cellar, hopefully the wine will be spared the worst of it. The greatest risk is that VSCs will be produced at the end of fermentation when CO2 production is slowing down, and won’t get blown off.
While there’s still debate among researchers about optimal YAN concentration, the number usually thrown around as the bare minimum for efficient fermentation and minimal VSC production is 140 mg N/L. Higher values are recommended, however, especially for grape musts with high °Brix.

Carbohydrates
The yeast generate most of their energy from metabolism of carbohydrates (sugars). Ethanol, higher alcohols, fatty acids and esters, all of which are important to the sensory perception of wine, are also derived from sugar metabolism. The good news is that grape juice typically consists of more than 20% easily fermentable sugar by weight, which is usually enough to produce a wine with around 12% alcohol by volume (ABV). The carbohydrate composition of grape juice is made up primarily of glucose and fructose occurring in roughly equal quantities, and while yeast will utilize both sugars, glucose is utilized preferentially to fructose.
Despite the abundance of sugar present in the juice, there are times when you may need to add additional sugar. For example, grapes grown in a cool climate, may have lower than desired sugar levels for winemaking. The most common reason to add sugar to juice (a process sometimes called chaptalization) is to increase the potential alcohol level in the wine.
Yeast prefer glucose over fructose, making glucose the best choice for increasing the fermentable carbohydrates. Sucrose, or table sugar, which is comprised of equal parts glucose and fructose, is the next best option for increasing potential alcohol. It can also be good for back sweetening. Fructose, being slightly less preferred by yeast, is not a great choice to increase the potential alcohol, but it does work well for back sweetening. Not only will it will make the finished wine slightly more stable, but in addition, you can use less of it to get the same sweetness because it is about 1.7 times sweeter than table sugar.
If you are adding sugar before fermentation to increase the potential alcohol, a good estimate is that about 60% of the sugar will be converted to alcohol. To get a 1% increase in potential alcohol you will need to add 17 g/L of a fermentable carbohydrate; glucose or sucrose would be ideal.
Oxygen
Oxygen is often considered bad news for wine production, especially for white wine, and oxygen exclusion is of paramount concern after fermentation to avoid browning and the growth of spoilage microorganisms. However, at the early stages of fermentation yeast require small amounts of oxygen to make essential cell components like unsaturated fatty acids and sterols, which help maintain the fluidity of the cell membrane. Generally, enough oxygen is incorporated into the juice during crushing and pouring into the fermentation vessel, but sometimes the dissolved oxygen can become very low, especially in highly-clarified juice. In such cases, simply stirring the must during reinoculation can be an effective strategy to incorporate a little oxygen.
Vitamins
Vitamins are a diverse group of compounds that help facilitate essential metabolic reactions. The function of vitamins can range from catalyzing reactions to transporting compounds within the cell, to acting like hormones to regulate metabolism. The quality that unites vitamins is that they cannot be synthesized in sufficient quantities by an organism, and therefore need to be obtained from the environment. Vitamin concentrations decrease during fermentation, and sometimes quantities are low enough that supplementation is necessary. Vitamin deficiency is rare in musts, however, and vitamins should be used sparingly as extra nutrients can encourage the growth of spoilage organisms after the fermentation is complete.
Minerals
Minerals are micronutrients that, like vitamins, are required in small amounts to facilitate biochemical reactions. Also like vitamins, these can be supplemented, but in most situations they are present in sufficient quantities.
Supplementation Strategies
Now that we have reviewed the classes of nutrients, let’s discuss specific strategies of supplementation. First, let’s talk about the timing of nitrogen additions. If we add a lot of nitrogen at the beginning of fermentation, it will encourage the yeast to replicate, thereby producing lots of biomass. The population will continue to grow until the nitrogen becomes limited. The advantage of this approach is that larger populations will ferment faster (many hands makes light work) and be less likely to get stuck, but the flip side is that, with a large yeast population, nitrogen can be used up quickly, bringing cell reproduction to an abrupt halt. It may be helpful to think of the reproducing yeast as a factory making new yeast cells. They like to keep an inventory of things they need on hand, but when they run out of a key component (nitrogen) they stop production and get rid of their excess supplies. Many of the excess supplies are precursors to amino acids for making proteins. Unused α-keto acids, which are amino acids without the amine (nitrogen) group, become fusel alcohols and acetate esters. Fusel alcohols, or higher alcohols, can contribute to complexity at low levels, but at higher levels give a harsh or solvent-like aroma. Esters usually occur at low levels, but are very potent and are described as fruity. Yeasts also keep stores of hydrogen sulfide (H2S) to make the amino acids cysteine and methionine. Unfortunately, H2S is the most potent VSC, with a very low detection threshold and an unpleasant rotten egg smell. One way to avoid VSC production is to add nitrogen throughout the fermentation. The recommended strategy is to add half of the nitrogen at the beginning of fermentation and the balance at the point where one third of the sugar is depleted. This ensures that the yeast will be well fed throughout the fermentation.
Types of Supplements
Next we will get into what kind of supplements to add. There are two basic types of assimilable nitrogen, inorganic nitrogen and primary amino nitrogen (PAN). Inorganic nitrogen is sold as diammonium phosphate (DAP), which is 21% nitrogen, and is the least expensive form of nitrogen that yeast will utilize. PAN is derived from yeast extracts. It is typically sold as a proprietary blend, and is more costly. There are a number of different blends, but most are no more than 4.2% nitrogen. Fermaid O® is an example of a product that contains only PAN. Rehydration nutrients, like Go-Ferm® contain small amounts of PAN plus other micronutrients to help keep yeast healthy during the early stages of fermentation. The final category of nitrogen supplements is usually referred to as complete yeast nutrients. These supplements typically contain a combination of DAP, PAN and other vitamins and minerals. Such blends are usually about 10% nitrogen; Fermaid K® is an example of this type of blend.
Timing of Additions
Now that we are familiar with the major types of yeast nutrients, let’s get into the nitty-gritty of what to add and the timing of additions. Before any nutrient additions are made, though, it’s important to know what you have to begin with. Characterizing the must to the best of your ability will help you figure out where you stand. At a minimum you want to know the °Brix, pH, and titratable acidity (TA), although measuring YAN would be ideal. Without knowing initial nitrogen status, it’s hard to know exactly how much nutrient to add. Since YAN measurements are somewhere between difficult and impossible to measure for home winemakers, the following recommend-ation is for prophylactic additions when YAN is unknown. Most of my experiences come from the cool climate region of New York’s Finger Lakes, where our YAN levels tend to be on the low side. This is especially so for cool climate vinifera varieties like Riesling and Cabernet Franc where the average YAN can be as low as 90 mg N/L. Compare these levels to fruit from the West Coast where YAN values tend to be closer to 250 mg N/L. Because of my experiences I may be inclined to add more nitrogen than others would recommend.
The first option is to add only DAP at the maximum legal limit (for commercial wines) at the beginning of the fermentation. This has been successful in fermentations where the varietal character was not of great importance and a fast fermentation was desired. The second option combines DAP and a complete yeast nutrient that will provide a small amount of PAN and some vitamins and minerals. Option 3 uses a rehydration nutrient which is a good way to get yeast started, especially if the conditions are particularly stressful such as cold temperatures or high °Brix. The fourth option combines rehydration nutrient with DAP and a complete yeast nutrient to give your yeast everything they could possibly need. Additionally there is a fifth option . . . which is to add nothing to your juice.
The five options described above are nutrient regimes that I have seen used in commercial operations, as well as in small scale production. I don’t think any of these are the only “right” way to go and there are certainly other options. The companies that sell yeast nutrients can also be good sources of information; Scott Laboratories (http://www.scottlab.com/) publishes a Fermentation Handbook every year with information about the products they sell as well as guides for how to use them effectively. There are endless combinations of nutrients that you can add, the best advice is to experiment.
What’s Next in Nutrition?
Here in the lab, I am conducting research aimed at understanding how nutrients in the must effect aromas in the wine. Amino acids can be transformed by yeast into fusel alcohols and acetate esters; differences in amino acid concentrations between grape varieties may be responsible for some of the differences in the aromatic character of the wine. Yeast can also produce esters via metabolism of sugar. Figure 1 shows how these two pathways are related.
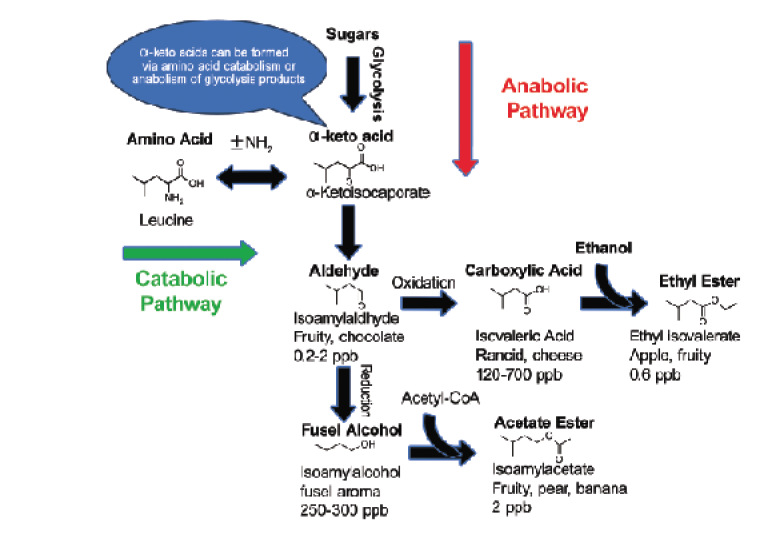
My research involves understanding how available nutrients affect yeast metabolism and the production of aroma compounds. One goal of the project is to determine how the ratio of ammonia to primary amino nitrogen (PAN) affects the aromatic properties of the wine so winemakers can make more informed decisions about supplementation. This research may also lead to the development of nutrient blends that will encourage the yeast to produce specific volatile compounds so that winemakers can have more control over the aromas produced in their fermentations.
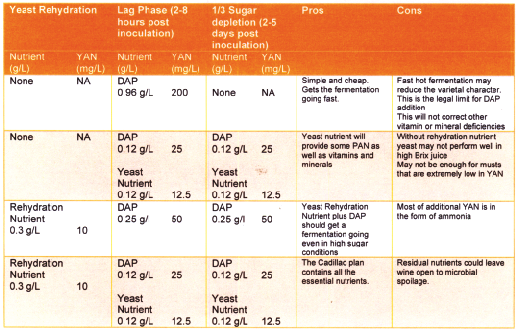
As with most parts of winemaking, nutrient additions can be as simple or complex as you choose to make it. The best piece of advice is to first do no harm; be judicious with your additions and think about the potential unintended consequences such as microbial instability. The other advice is to experiment with different approaches and determine what works best for you. It helps to keep a fermentation log that includes information about what you tried — including the type of nutrient used, how much you added and when — and the results you observed. Record both the details of your fermentation — how long it took, the final hydrometer reading, any unusual odors coming out of the air lock, etc. as well as tasting notes of your wine. Depending on the volume of wine you make, you may have the opportunity to conduct an informal experiment. For example, if you have two carboys of the same juice, try using a different yeast nutrient plan for each.






