
I hope I may be forgiven for the technical nature of this article. Alas, my experiences over the last 30 years have granted me a rich body of technical distinctions without which the subject of volatile acidity is rife with misconceptions, bad theories, and poor decisions. Here I will hold your hand as we descend into this age-old enological murk in hopes to emerge with useful principles and protocols related to this wine fault.
Volatile acidity (VA) is a euphemism for vinegar. In conversation, it generally includes the ester ethyl acetate, the compound mostly responsible for the vinegar smell. Acetic acid itself is not easily detected in wine by nose. When people smell VA in wine, it is most often ethyl acetate rather than acetic acid they smell. What we actually measure in a cash still1 does not include ethyl acetate but does include small amounts of lactic acid. A more precise and specific measure of acetic acid can be obtained enzymatically.
Acetic acid and ethyl acetate in wine smell a little different than vinegar due to the presence of alcohol. In distilled white vinegar, acetic acid has a pleasant, sweet smell. In wine it has practically no smell due to its alcohol solubility. Ethyl acetate in nail polish remover has an aggressive chemically solvent smell, whereas in wine, its smell has a sweet aroma you’ll see in red wine vinegars.
The main sensory property of acetic acid is a sour taste in the wine’s finish. Its aroma in the concentrations present in wine is nearly undetectable, aided also by its solubility in alcoholic solution. The threshold for acetic acid varies greatly depending on the tannin structure in the mouth. It can appear as a metallic taste in the finish of white wines at around 0.6 g/L but is often undetectable at twice this concentration in big Cabernets and other highly structured red wines.
In 1981, master winemaker John Franzia addressed our class at UC Davis, claiming that he knew how to address every possible wine flaw except VA. Over a decade later in 1992, I invented a process for removing volatile acidity from wine2 using re-combinatory reverse osmosis and ion exchange. Over the next 17 years my company Vinovation, Inc. (which I have since sold to Winesecrets) processed 35,000 high-VA lots.
When done correctly, the process was extremely successful and we were able to help put several hundred wines on the Wine Spectator list of the top 100 wines of the world.
Before we came along, VA was held to be a relatively rare phenomenon caused by negligence in the cellar. Winemakers shamefully kept it to themselves. When we queried winery labs and bulk wine brokers, their estimates ranged from 30,000 to 80,000 gallons annually impacted by VA in California. We now know that volume is about 2 million gallons per annum. And almost none of it is caused by negligence in the cellar.
The Usual Suspects
So, if winemaker negligence isn’t the major factor causing VA, what is? Most often, it starts in the vineyard. Five genera that produce VA are shown in Figure 1.³
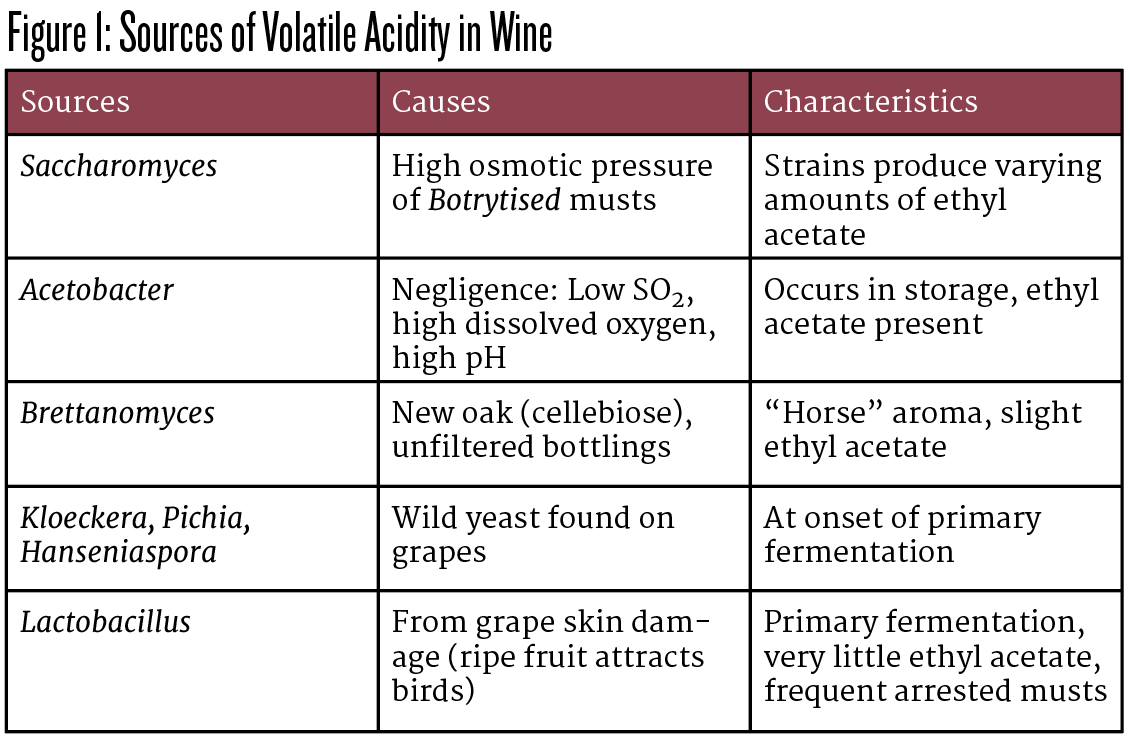
The lion’s share of wines we treated over those 17 years at Vinovation were bird-damaged fruit carrying infections into the fermentation tank where highly virulent bacteria, largely the newly discovered Lactobacillus kunkeei, would rapidly grow to 109 cells per mL and overnight convert sugar directly to acetic acid in excess of the legal limit without any oxygen present. These bacteria are so numerous that you can actually see a telltale iridescent sheen on the must’s surface.
Grapes exist to attract birds — they use avian vectors to spread their seeds. Bird damage favors the best vineyards — those with the most color and flavor — so we ended up processing mostly quite expensive wines. Fermentative VA doesn’t make ethyl acetate, so the wines generally smelled fine.
About 20% of these wines would quit fermenting prior to completion, and removing the acetic acid usually allowed them to finish fermentation and go dry. We published a study performed at Napa Valley College showing this effect.4
Prevention
The prevention of VA in red wine is one of the most misunderstood aspects of winemaking. For the full rundown, please read my article “Sulfur Dioxide: Fact and Myth” in the February-March 2023 issue.
For many years, advice to prevent VA always focused on preventing negligence in the cellar leading to oxidative VA caused by Acetobacter. In whites, thorough gassing of receiving carboys or tanks prior to racking into them, the use of dry ice in the receiving vessel to create a blanketing layer, maintaining molecular SO2 at 0.8 mg/L are the standard mantras.
Reds are a bit trickier. Most or all so-called free SO2 in red wines is bound to anthocyanins.5 Anthocyanin-bound SO2 is ineffective in controlling Acetobacter.6
The good news is that healthy reds have good oxygen appetites, which is to say that their reactive phenolic constituents hungrily consume oxygen during oxidative polymerization. Anti-oxidative strength is essential to controlling Acetobacter, which requires dissolved oxygen to thrive. By healthy, I mean harvested ripe but not overripe. Excessive ripeness diminishes oxygen appetite by as much as 90%.
Phenolic oxygen consumption is maximized at 59–65 °F (15–18 °C) and is diminished by 80% at 50 °F (10 °C), so cold cellars encourage VA production. Think Bourgogne, with many high-VA wines.
Because of its heavy pigmentation and the resulting binding of SO2, Norton is particularly prone to Acetobacter spoilage, and we routinely dose it with chitosan during aging. Chitosan is a wonderful innovation derived from chitin, the material that makes up the carapace of shellfish and the structure of mushrooms. Chitosan is a particulate form that ruptures and precipitates cell membranes of yeast and bacteria. Several suppliers sell it.
The bizarre heat spell in early September 2022 in California robbed our fruit of oxygen appetite, producing the worst year in history for VA. To prevent this, we quickly learned to add chitosan and antioxidants.
What about the other sources of VA? Bird-damaged grapes or fruit otherwise afflicted with sour-rot can be sprayed on the vine or at the crusher with the bioprotective yeast Metchnikowia pulcherima, which suppresses spoilage yeast and bacteria. In the fermenter, lysozyme is effective against Lactobacillus kunkeei.

How RO / AIX Works
Next I’d like to talk about treatment and how small operations, even home winemakers, can band together to acquire this capability. Shared equipment is one of many benefits of being part of a home winemaking club. Since the removal of volatile acidity is a common need and the required equipment is beyond the means of most single practitioners, joint ownership of a reverse osmosis unit is the most practical means to gain access, as these units cost over $10,000.
Almost all wine acids are ionized at wine pH. Ions are not volatile — they cannot evaporate into the gas phase and therefore do not contribute to aroma. The exception is acetic acid, which ionizes at pH 4.8 and is almost entirely present in the uncharged molecular form in the wine range between pH 3.0 and pH 4.0.
Charged ions attract water molecules of hydration, increasing their functional molecular weight by 500 daltons or more. In contrast, molecular acetic acid, with a molecular weight (MW) of 60 daltons, is the only wine acid that can pass through an 80-dalton reverse osmosis membrane (e.g. the VinoCon RO-5), occurring in the permeate at about 60% of the concentration in the wine. This allows it to be completely adsorbed by a weak anion exchange resin.
Ethyl acetate (MW= 88) also passes into the permeate at 40% and is hydrolyzed by the high pH conditions in the column. The cleaned permeate is recombined into the wine, resulting in diminishment of the VA without any other removal of flavor or structure.
Since nearly all constituents that contribute to wine’s aroma are in the MW range between 100 and 200 daltons, it is critical for good results that membrane porosity below this range are employed.
Doing it Right
If your group is purchasing a reverse osmosis unit, very tight membranes are essential to making a clean separation of acetic acid from all other wine components. The best systems operate at 1,000 PSI, so the resulting 800+ PSI pressure differential optimizes permeate flow and sharpens the separation. This type of RO can also be used to reduce alcohol without harming flavor. Since the osmotic pressure of 20 °Brix juice is about 600 PSI, 1,000 PSI machines are popular in France and the Eastern U.S. to increase must Brix by removing rainwater.
The second essential is the selection of a weak base anion resin (I use Purolite 103a). This type of resin has tertiary amino groups to which the acetic acid clings rather than exchanges. Wine pH is thus unaffected. Resins are regenerated with base: Liquid KOH or NaOH are employed in professional systems, though in amateur operations, non-chlorinated trisodium phophate (TSP) is safer.
In order to cleave ethyl acetate (the odor of vinegar or varnish at high concentrations), keep your exit pH above 9.0. This means more frequent recharging. Otherwise, you can run all the way to 6.0 if acetic acid removal (the sour taste in the finish) is your only aim.
When a lot is finished processing, there may be color and flavor hung up on the membrane that you’ll want to dislodge and return to the wine. This is done by pushing the permeate out of the resin canister and into the upstream side of the filter.
Done correctly, you will experience no change in wine composition or volume. It’s an art.
Doing it Wrong
Buyer beware. Some industry RO units on the market violate these principles. The higher the porosity of the membrane, the lower the machine’s running pressure needs to be. Their strategy is to utilize membrane porosities as high as 300 daltons, allowing cheaper machines to be built that produce high permeate flows at pressures as low as 600 or even 400 PSI. Obviously, these loose membranes are going to strip considerable flavor and texture. It gets worse. Since these loose membranes strip substantial amounts of tartaric, malic, and lactic acids, strong base anion exchange resins are employed. These are not adsorbent, but rather swap acetate ions of hydroxide, which is then added to your wine, typically resulting in a pH shift as much as an entire point, e.g. from 3.6 to 4.6. Believe it or not, the protocol then asks you to correct this shift using hydrochloric acid (HCl).
These vendors generally will not tell you the pore size of the membranes they are using. Then the operating pressure will be low. The strong anion exchange resins will have a strong fishy odor, and the protocol will involve adding mineral acid.
Latest Innovations
Now here’s exciting news. Dr. Alice Vilela-Moura’s team at Institute for Biotechnology and Bioengineering in Portugal has developed a protocol to use yeast to reduce VA in musts and wines. This process does not involve expensive equipment and apparently will work on a small scale. To my knowledge, this technique has yet to be commercialized, and I have never tried it. Several yeast strains have been successfully employed in musts and wines, and the most successful is referred to as Sacch. cerevisiae strain S26, which can substantially reduce acetic acid during fermentations of tainted wines mixed with fresh must.7 An additional technique is to immobilize lees from a recent fermentation on alginate beads and pass the wine through the bed.8
Dr. Vilela-Moura’s papers are easy to find on the Internet. They’ve been around for over a decade, and why they haven’t been commercialized puzzles me. I haven’t yet tracked down the magic yeast, but it would be a boon to small-lot winemakers. I’ve brought the paper to the attention of the major wine yeast suppliers, so keep your eyes peeled. I further hope that readers will be inspired to set up experiments contributing to our understanding of this exciting possibility.
References:
1 Cash Still operation https://www.youtube.com/watch?v=o4ITITtufDg
2 Smith, C. United States Patent 5,480,665. Apparatus and Method for Removing Compounds from a Solution. 1996.
3 Rodriguez, D. and C. Smith, Volatile Acidity Reduction of Wine via Selective Adsorption of Acetic Acid from Reverse Osmosis Permeate. OIV proceedings Expert Groupe sur la Technologie du Vin, 1999.
4 Smith, C. et al. Acetic Acid as a Causative Agent in Producing Stuck Fermentations. American Journal of Enology & Viticulture, Vol. 46, No. 2, 1995.
5 Patricia Howe, Re-Thinking Free and Molecular Sulfur Dioxide Measurements in Wine. Dissertation presented to the faculty of the Graduate School of Cornell University, 2015.
6 Aline Lonvaud-Funel, Les aspects microbiologiques de l’élevage des vins rouges en barriques. Colloque des Sciences et techniques de la Tonnellerie, 2000.
7 Vilela-Moura A, Schuller D, Mendes-Faia A, Côrte-Real M(2010 a) Effects of acetic acid, ethanol and SO2 on the removal of volatile acidity from acidic wines by two Saccharomyces cerevisiae commercial strains. Appl Microbiol Biotechnol 87:1317–1326.
8 Vilela, A. Appl Microbiol Biotechnol (2013) 97:4991-5000.






