
Chemical and microbial stability are a group of activities that occur after fermentation but prior to bottling that are critical to quality wine production. Once primary and malolactic fermentation are completed, chemical and microbial stability are the main concerns of the winemaker prior to final clarification, filtration, and packaging.
When “stability” is discussed in a wine, microbial stability is one part of this, while heat and cold stability (or protein and potassium bitartrate stability) is a different procedure that will be discussed in a future article. Heat and cold stability are most critical for white and rosé wines. Microbial stability, on the other hand, is a universal concern for all wine styles. In this article we’ll discuss the most common methods for achieving microbial stability, both during aging in the cellar and when packaging.
Microbial stability involves protecting the wine from microbial contamination and subsequent off aroma generation caused by microbial metabolism. Without human intervention the natural next step for wine is to be converted to vinegar by acetic acid bacteria. These bacteria require oxygen for metabolism, and chemical oxidation also negatively changes the aroma of wine, so step one in microbial stability is protecting the wine from oxygen. Wine storage vessels must be filled to the top or purged frequently with inert gas to prevent oxidation, and wine processing steps should be undertaken in a way that minimizes oxygen pickup as much as possible.
Unfortunately, acetic acid bacteria are not the only microbes we must be concerned about during the aging process between fermentation and bottling, so step two in microbial control usually involves the judicious and repeated use of potassium metabisulfite, or SO2. SO2 acts as both an antioxidant and antimicrobial agent, and this dual impact is why it is so difficult to make high-quality wine without the use of sulfites.
Before we discuss SO2 use in aging wines, a brief word about timing. Some winemakers like to utilize an SO2 addition at the crush pad, especially if the fruit has been damaged prior to harvest. The thinking here is that SO2 will inhibit native microbes on the fruit until Saccharomyces yeast are added and outcompete other microbes. Addition rates tend to be in the 25–100 ppm range, with dosage increasing with increasing fruit damage. All of the SO2 added at the crush pad for reds, or in the press pan for whites, will be bound and consumed relatively quickly, so this level of addition won’t inhibit Saccharomyces fermentation. It may, however, be inhibitory to malolactic fermentation since lactic acid bacteria are sensitive to both free and bound SO2. Therefore, SO2 use should be limited to 25 ppm or less prior to the completion of malolactic fermentation, if malolactic fermentation is desired. Finally, if possible, it’s important to check for both primary fermentation completion, and malolactic fermentation completion if desired, prior to the addition of more SO2. Wines with residual sugar and malic acid may be susceptible to further microbial metabolism and spoilage during aging, so understanding if these fermentation processes are complete is a very helpful tool in quality winemaking.
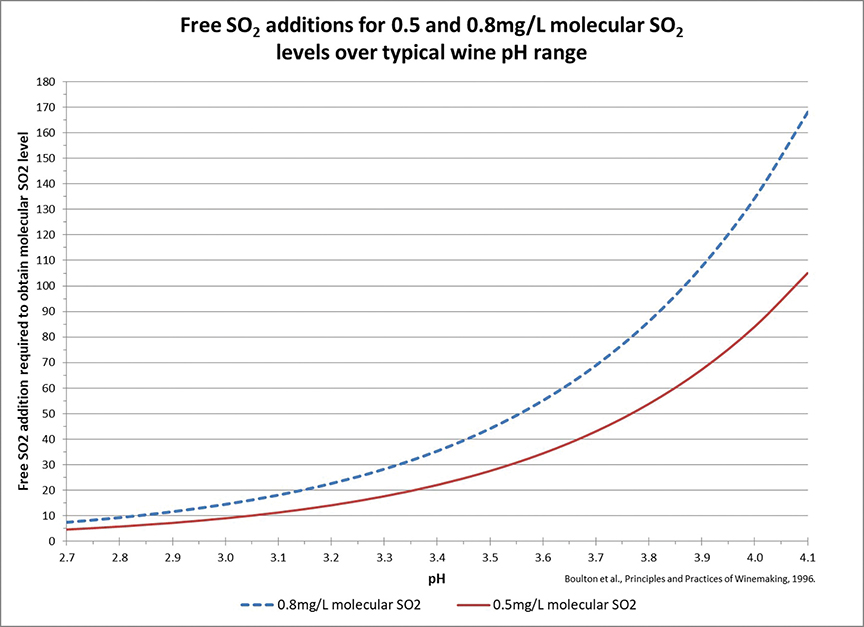
When SO2 is added to wine some of it is becomes bound to other molecules in the wine (acetaldehyde, anthocyanins, and sugar are common examples) and is thus unavailable to contribute to antimicrobial and antioxidation reactions. The remaining free SO2 dissociates into several forms when added to wine. The percentage of free SO2 in each form depends on the pH of the solution. The active form of free SO2 in terms of antimicrobial action is called molecular SO2. The percentage of free SO2 in the molecular form is much higher at pH 3.0 than at pH 4.0, thus to protect wine from microbial spoilage the level of free SO2 needed is lower at low pH, but very high at high pH.
One could write an entire article on SO2 use in wine (and, in fact, they have as there are already several good ones for further reference at winemakermag.com to review), so to keep this article to a manageable length we’ll cut right to the actionable information. Figure 1, below, displays a chart of the free SO2 level required at a particular pH to keep the molecular SO2 level between 0.5 and 0.8 ppm molecular SO2. During aging most winemakers try to keep the free SO2 level of their wines somewhere between the two curves to limit microbial metabolism in the wine.
At the levels we utilize SO2 in wine production it does not outright kill microbes, but inhibits their metabolism so that they cannot utilize other components in wine as an energy source. This is an important concept to understand because it means that if the SO2 level in wine drops below a certain threshold then some microbes, usually SO2-tolerant and ethanol-tolerant yeast strains, may become capable of metabolism and growth. One of the first commercial wines I produced was a rosé with about 1.5% residual sugar. My attempt at sanitizing the bottling line with iodine was inadequate, probably due to improper concentration or contact time, and even though the wine was stable for several months due to my SO2 addition prior to bottling, it inevitably ended up refermenting. The free SO2 level in the wine declined over time due to slow oxidation in bottle, and the carbon dioxide created by yeast metabolism was enough to push the corks out of the bottle. We had to recall the remaining bottles and reimburse several loyal customers who had sustained cleaning expenses from wine leakage.
My refermentation nightmare in the previous paragraph leads us to some general rules regarding microbial stability. If a wine contains appreciable amounts of sugar (greater than 3 g/L or 0.3%), and in some cases malic acid (greater than 0.3 g/L), then microbes must be eliminated in bottle or the wine will at some point become unstable. Conversely wines that are fermented dry and have gone through malolactic fermentation are usually safe to bottle without sterile filtration or the addition of chemical inhibitors. There are a few qualifiers in the previous sentences so let’s discuss some examples for specific wine styles.
Microbial Stability for White and Rosé Wines
White and rosé wines most often have not completed malolactic fermentation and the wines may contain some residual sugar to balance the acidity. It’s worth noting that winemakers may have difficulty determining whether residual sugar is present in any wine style, white or red, because it is impossible to determine the residual sugar level by measuring °Brix or specific gravity alone. While a stable °Brix measurement over several days at a level somewhere around -1 °Brix is an indicator of fermentation completion, it may also mean that the fermentation has stalled at a low residual sugar level. The only way to know for certain is to measure the sugar content. The sugar pill test (e.g., Clinitest®) is one method accessible to home winemakers. This test works by the action of sugar molecules reducing iron ions in the pill to create a color change. In my experience the test is reasonably accurate but does sometimes indicate small amounts of residual sugar when there is actually none, because some wines contain small quantities of reducing agents other than sugar.
For malic acid in wine, as mentioned previously, lactic acid bacteria are inhibited by higher levels of free and total SO2. So for standard wines at greater than 10% ABV, if total SO2 levels are greater than 50 ppm then lactic acid bacteria should be inhibited and are thus not a big concern.
Sterile Filtration
Sterile filtration is a common commercial method of eliminating microbes in bottled wines that contain residual sugar, where the filter media is small enough and uniform enough that none of the yeast or bacteria that could cause problems in finished wine are allowed to pass through the filter. A 0.45 µm membrane cartridge filter and filter housing are required. Nominally rated “sterile” pad filters made of cellulose are not designed to completely guarantee removal of all microbes, thus sterile pads in a plate-and-frame filter are a good final pre-filtration option, but are not an equivalent alternative option for a sterile membrane cartridge filter.
Sterile-filtered wines must be pre-filtered prior to passing through the sterile membrane or the filter will quickly clog. In addition the filter and all piping to the bottle must be sterilized prior to bottling. Commercial winemakers use 180 °F (82 °C) water, steam, or ozone to sterilize their bottling lines, options not available to most home winemakers. Since home wine bottling is done on a small scale, it is possible to sanitize bottling equipment with iodine, 70% ethanol, or an acid-based sanitizer such as Star San. When using these chemical sanitizers one must follow the dilution ratio and contact time (usually a minute or two) recommendations from the manufacturer for the sanitizer to be effective. It’s also important to note when sanitizing that the bottling equipment must be free of soils (i.e., clean, with any solids and biofilms removed) prior to sanitizing in order for the sanitizer to be effective.
Chemical Inhibition

Potassium sorbate is a chemical inhibition option for home winemakers, and is sometimes used commercially in sweet wine production. Sorbic acid is actually the inhibitory compound, but potassium sorbate is more soluble than sorbic acid, thus the sorbate salt is added and the sorbate ion dissociates from the potassium ion once added to the wine. Sorbate is usually added at 100–200 mg/L sorbic acid, depending on the alcohol content (more is needed for lower alcohol levels). Potassium sorbate is about 75% sorbate by weight, so this should be taken into account when calculating the addition rate. Sorbate inhibits yeast metabolism only, so SO2 is still required for bacterial inhibition. In addition, lactic acid bacteria commonly employed for malolactic fermentation can metabolize sorbic acid to produce a fault known as geranium taint, because the aroma is reminiscent of geranium leaves, so SO2 must be utilized to prevent bacterial metabolism when sorbic acid is used. There is some research suggesting that sorbic acid is converted to ethyl sorbate over time, which has been described as having both vegetal and tropical fruit aromas. Perhaps this is why commercial producers only use sorbate on very aromatic dessert wines.
Commercial winemakers also have the option of adding a chemical compound called dimethyl dicarbonate (DMDC). DMDC is effective against both yeasts and bacteria, although it is less effective against high levels of bacterial contamination so use of SO2 is still recommended. DMDC is unstable in wine and several hours after bottling it breaks down into harmless levels of CO2 and methanol. Unfortunately, DMDC is also extremely toxic to humans and thus requires an expensive dosing system for safe integration into a bottling line, which puts this chemical inhibition method out of reach for home wine producers.
Microbial Stability for Red Wines

For most red wines, where the wine has been fermented to dryness and malolactic fermentation has been completed, it is usually possible to bottle microbially-stable wines without sterile filtration or chemical inhibition. The exception is wines containing the yeast Brettanomyces, colloquially referred to as Brett. Brett can continue to metabolize and grow, even in dry wines without residual sugar or malic acid. There is some research suggesting that Brett can metabolize odd-ball sugars that other yeast do not utilize (like pentoses) or that Brett may metabolize ethanol itself. When Brett grows it creates a host of volatile organic chemicals that create a range of aromas, from Band-Aid or hospital sanitizer, to barbecue, to sweaty horse aromas. But Brett also tends to be a slow grower in bottled wines, perhaps due to the lack of energy sources. This can lead to significant bottle-to-bottle variation; where some bottles smell like the original wine but others smell significantly of Brett metabolites. Some people enjoy the aromas produced by Brett, it can be found in both cheap and expensive wines, as well as some styles of beer and cider. Others find the aromas off-putting and commercial winemakers in the U.S. often think of wines containing Brett to be flawed. My opinion is that a little Brett aroma is acceptable, depending on style and the other components in the wine, but when Brett aromas overpower all other wine aromatics it makes a wine one-dimensional.
If red wines have residual sugar or malic acid the same rules apply as those for white and rosé wines: Moderate amounts of SO2 must be added to inhibit lactic acid bacteria and the wine should be sterile filtered or chemically inhibited if residual sugar remains. One final exception to these rules might be fortified dessert style wines. If the alcohol content has been raised above 18% ABV by addition of grape brandy or other fortifier then it is unnecessary to be concerned about further microbial metabolism, no matter the residual sugar level. The microbial stability afforded by fortification is a big benefit for this wine style if sweet wines are your preferred tipple.
Pulling it all Together
While we didn’t spend much time discussing it, it’s important to remember that oxidation and microbial spoilage can happen independently, but most often happen in tandem since high oxygen levels quickly deplete any free SO2 in the wine. Spoiled wines with high levels of acetic acid are by definition oxidized, and besides the aromas of vinegar and nail polish remover they also lose their fruity aroma and their color shifts toward brick red or brown. However, I’ve also made and tasted wines that have been protected from microbial spoilage by SO2 use, but nonetheless have the oxidized aroma of over-ripe apple and have lost their fruity aromatics due to poor storage conditions where chemical oxidation occurred.
The natural wine movement has been increasing in popularity and is focused on both the limitation of additives as well as the low or no use of SO2. It’s certainly possible to make wines without SO2, but in my opinion very clean cellar practices and a somewhat religious zeal for limiting oxygen pickup are required to achieve quality results. Natural winemaking is also most successful in red or fortified wine production where higher alcohol levels and tannins in the wine can act as the antimicrobial and antioxidative agents. Wines with low or no SO2 use are also usually aged for a shorter period of time prior to bottling and are best enjoyed young.
Developing a game plan for maintaining microbial stability prior to harvest can be helpful, since activities can become hectic once production activities get underway. Things to consider when coming up with a plan are: Fruit quality, wine style, SO2 usage concerns, malolactic fermentation completion, aging plan, and packaging ability.







