Practical Sulfite Management

In 14 years of assisting home winemakers in a beer and winemaking shop, questions about sulfites came up often. While some hobbyists were bound and determined to use no sulfites in their wine, most wanted practical advice to help produce a sound, pleasant, drinkable wine in the finished bottle. While “no sulfites” is at least a simple program to apply, it has never been my choice as the most reliable way to make good wine. Lots of theoretical information is out there — and we will necessarily touch on a bit of theory today — but most practical is a simple, prescriptive approach that has long proven to be almost always successful. That is what I am describing here.
To be clear on concepts and terminology, we will cover some basic facts about sulfur. Chemically speaking, sulfur is a reactive element that can adopt a range of oxidation states. The dreaded “rotten egg” smell of hydrogen sulfide, H2S, represents one end of the oxidation scale with sulfur in the minus two oxidation state. At the other end of the scale are some sulfur compounds in plus oxidation states. One of these is today’s subject: Sulfite. Sulfite refers to the ionic form in aqueous solution (water or wine) that is noted by the symbol SO32-. Depending on the pH of the solution, a portion of sulfite ion combines with a hydrogen ion, H+, to form the bisulfite ion, HSO3–. The reason you hear sulfite additions sometimes called SO2 additions is because that compound, sulfur dioxide, is a gas that reacts to form sulfite and bisulfite ions when it is dissolved in wine. An important note is that in acidic (low pH) solutions, a tiny fraction of the sulfite also occurs in the molecular form, dissolved sulfur dioxide, regardless of how it was introduced to the wine. While you may hear a winemaker say they add “sulfur” to their wine, it is only meaningful if they are adding sulfite. Commercial winemakers may actually add sulfur dioxide gas, but that material is too hazardous for home use. We use other forms of sulfite.
Before details or “how,” here is a little bit of “why.” For my practical program, I apply a consensus approach that I have heard from many commercial winemakers. While it may not have exact theoretical justification, it seems to work well. The target under this program is to maintain 0.5 mg/L (parts per million, ppm) of molecular sulfur dioxide in red wines and 0.8 ppm in white and rosé wines. At those levels, the added sulfite helps protect against oxidation and browning of the wine while also inhibiting the growth of harmful microorganisms. One drawback to those cited levels is that we do not have practical means of analysis for molecular sulfur dioxide in wine. However, there is a well-understood relationship between the amount of molecular sulfur dioxide and the sum of sulfite and bisulfite ions at a given pH. That sum is referenced as “free” SO2, and we do have reliable analytical methods for it. Since the ratio is pH-dependent, there are various calculator tools and charts to help interpret it. One such calculator is at Winemakermag.com/sulfitecalculator. Table 1, below, illustrates some of the ratios at the 0.5 and 0.8 ppm levels.
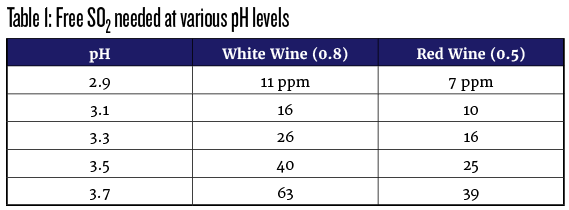
Along with guiding sulfite additions, this table is a good reminder of why we try to keep pH low in our home winemaking. Since se nsitive tasters can begin to detect the presence of sulfite at about 50 ppm, it may be difficult to produce a high-pH wine without suffering an unpleasant “burnt match” aroma or giving up some level of protection.
For initial additions of sulfite to your wine, you can simply add the recommended amount as dictated by the pH. But how will you do that? Home winemakers use various forms of the solid material potassium metabisulfite, chemical formula K2S2O5, which dissociates in water (or wine) to produce two positive potassium ions and two negative sulfite ions. The sulfite ions distribute into sulfite, bisulfite, and molecular sulfur dioxide according to the pH of the solution. While sodium metabisulfite (Na2S2O5) would work the same way, we do not want to introduce sodium salts to our wines that might be harmful to a person on a sodium-restricted diet.
Potassium metabisulfite goes by several nicknames in winemaking: Pot meta, KMBS, KaMBuS, Campden tablets, and sometimes (confusingly) just “sulfur.” We will stick with its full name or chemical formula here to remain perfectly clear, but be aware that you may hear these other names. If you work with the powder itself, a key factor is that a given weight of potassium metabisulfite yields 57.6% of that weight as sulfur dioxide when it is dissolved in water. That is, adding 100 g of the solid produces the same final concentration as adding 57.6 g of the gas. These ratios come about for two reasons. The first is that the potassium ions play no role in the sulfite concentration and are just along for the ride. The second is that chemical term “meta” in the compound’s name. In this chemical context it means something like “about to become.” That is because the S2O52- produces the equivalent of two SO2 molecules upon dissolving, those react to form (partly) bisulfite, so the solid was “about to become” potassium bisulfite when dissolved in water or wine. The important takeaway is that you get 57.6 g of sulfur dioxide out of every 100 g of potassium metabisulfite.
Here are some ways you can use potassium metabisulfite in your wine program. To help with comparisons, each method is given with an addition to a 5-gallon (19-L) carboy of wine. Using the powder itself, if you add one gram of potassium metabisulfite you are adding 576 mg of SO2. That 576 mg in 19 L is an addition of 30.3 mg/L. It is much less accurate to measure a powder by volume, but if you don’t have a small scale to weigh gram quantities, it may be your best choice. At the midrange of 1.25 g/mL a teaspoon (5 mL) of potassium metabisulfite powder weighs about 6 g or a quarter teaspoon contains about 1.5 g. You can use that weight estimate to make a corresponding addition. A quarter teaspoon in 5 gallons (19 L) is about 45 ppm (1.5 x 30.3 ppm).

Campden tablets are a pre-measured form of potassium metabisulfite compressed into a pill that needs to be crushed and stirred into a bit of water or wine before you add it. They are most commonly sold at a size that produces 65 mg/L when added to one gallon (3.8 L) of wine. To continue our comparison of 5-gallon (19-L) volumes, one tablet crushed and added to that carboy would introduce 13 mg/L of SO2. Note that you do not need to account for the difference between the weight of potassium metabisulfite and the yield of SO2, as the tablet manufacturer has already taken that into account. Check your package label when you buy Campden tablets as other sizes are sometimes produced.
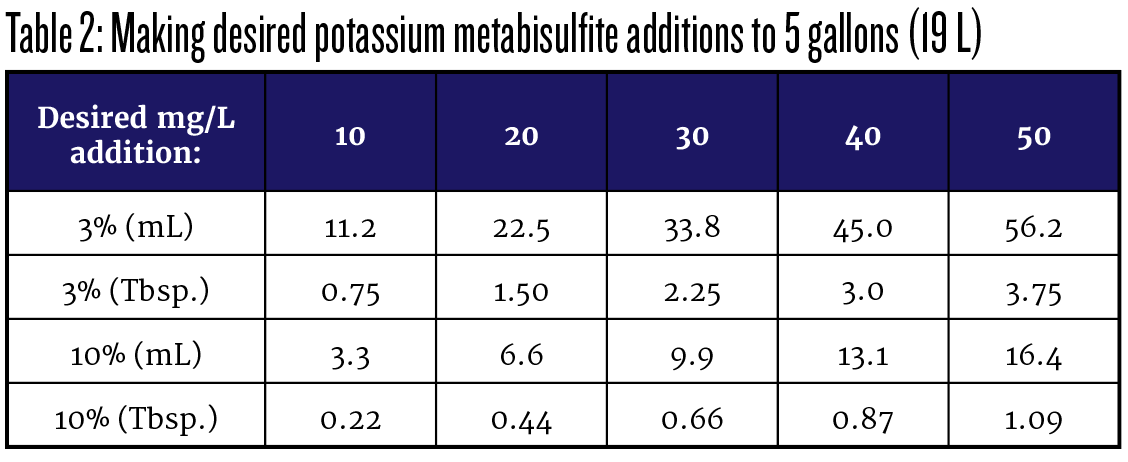
With stirring often recommended for solid additions of potassium metabisulfite, I prefer to work with a solution so I can make the addition quickly and re-stopper the carboy or barrel with less air exposure. For small quantities of wine, it is convenient to make a 3% solution. You can purchase potassium metabisulfite powder in a 4-oz (113-g) bag and dissolve the entire contents in a gallon (3.8 L) jug of distilled water. Label it “3% Potassium Metabisulfite,” add the date and mark it “Poison” and you are ready to go. If you are making larger quantities of wine, you may find a stronger 10% solution suits your needs better. To make that, dissolve 100 g of potassium metabisulfite powder in about 900 mL of distilled water. Dilute up to exactly one liter, label as “10% Potassium Metabisulfite” plus the date and “Poison.” Note that these percentage figures, 3% and 10%, reference the whole powder, so the SO2 content is, as noted earlier, 57.6% of that value. A 3% solution contains 0.03 x 57.6 or 1.7% SO2 and a 10% solution contains 5.76% SO2. From those values you can calculate your needed addition to any volume of wine, or you may find it quick and convenient to use a reference chart. A graduated 10-mL pipet is convenient for adding portions of 10% solution while common kitchen tablespoons (15 mL each) work well for 3% solution. Table 2 is filled out for additions to 5 gallons (19 L) of wine, using either tablespoons (Tbsp.) or milliliters (mL).
There is another method only suitable for home winemakers who are making larger quantities of wine (even in its smallest dose it is not suitable for 5 gallons/19 L). This method can be especially useful on harvest day and uses pre-measured pills labeled as “effervescent tablets.” The tablet is made of potassium metabisulfite with a very small amount of potassium bicarbonate blended in. As a result, when the tablet enters an acidic medium like must or wine, it effervesces and completely dissolves all by itself. Like Campden tablets, they are labeled in yield of SO2, not actual tablet mass. They come in 2-gram and 5-gram sizes, packed in a three-tablet blister pack. A 2-gram tablet, containing 2,000 mg of SO2, would add 105 mg/L to a 5-gallon (19-L) carboy — more than you would ever want to add. However, if you are sulfiting your must at crush on harvest day, adding a 2-gram tablet to 20 gallons (76 L) of must in a food-grade trash can, the addition is just 26 mg/L, and it is very convenient to use.
Sulfite Additions
Now that you have your chosen method of addition, what is the program? Add 50 ppm of sulfite to the freshly crushed must. I crush my grapes and destem into 32-gallon (121-L) square bins, so I estimate each one as having a bit over 20 gallons (76 L), having left enough room for cap rise later. (I fill the bins with white grapes to about the same level for ease of handling.) When a bin reaches half full, I toss in one 2-gram Inodose Effervescent Tablet. When the bin is full, in goes another tablet and I stir the must with a wooden paddle. That gives me about a 50-ppm addition. There is no need to test for sulfite after this addition as it will all be used up by the time fermentation is underway. You can add a lower dose or, if the fruit is damaged or moldy, a higher dose — but 50 ppm works well for me vintage after vintage.
No more sulfite additions are made until fermentation is complete. For white wine, rack the finished wine off of the gross lees. Unless you will do malolactic fermentation (MLF) on your white wine, add another 50 ppm of sulfite. Use a sulfite calculator and your pH to determine your longer-term aging target level, but 50 ppm added will be a good start. For red, press the must and, if desired, inoculate for MLF. After MLF is complete, or if you are not doing MLF, add 50 ppm here also. You may delay this addition for a day or two after pressing in non-MLF reds since pressing might release a bit more sugar and primary fermentation may resume briefly. When the wine is completely dry, make the sulfite addition. Once again, look up your aging target level. After a week or so to allow initial reactions to quickly use up whatever sulfite they need, test for free SO2. Subtract how much you have from your target level and calculate an addition to make up the difference. You can safely round off a bit here to make the addition convenient, since you have months of aging ahead of you.
My experience has shown that aging new wine loses about ½ part per million per day. So, in about 30 days, expect to have a deficit of about 15 ppm. Make that addition after one month of aging, wait a day or two for reactions to occur, then test again. Make any adjustment that still may be needed. After the second month, add another 15 ppm. You can test again if you want to, but I usually skip a month of testing, then add another 15 ppm after the third month, test, and adjust. Losses will probably slow down during aging. If you are above your target on one test, don’t worry about it. You will drop back down by next month. If you are concerned about going over, you can always test before making an addition, but then you will need to test again after the addition to know what you really have. Just because you added it, does not mean it will show up as free SO2. Various reactions are using it up throughout the bulk-aging period.
Check again to make sure you are on target when you get close to bottling. If a high pH has had you maintaining much above 30 ppm, you may want to let it drop some before bottling. As noted earlier, sensitive tasters are able to detect about 50 ppm of sulfite in the wine. If you bottle at that level or higher you may need to age for a few weeks or months to bring the wine back to a pleasant level of undetectable sulfite. When levels are not too high, and since air exposure is inevitable when we bottle wines at home, I like to make a final 10 ppm addition to the bottling bucket or tank to counteract that final oxygen input to the wine. Put the cork in the bottle and your sulfite program is done!
Testing for Sulfite
When doing the recommended testing for free SO2, you have a few method choices. On one end of the scale is the Ripper method, a color-endpoint iodine titration that you can carry out with laboratory apparatus on a home-wine basis. It is messy and takes some practice to see the endpoint, especially for red wine. There is a packaged kit employing the Ripper method called Titrets that uses a pre-measured vial for each sample. It is recommended only for white wine.
Another laboratory-grade method for sulfites that you can do at home with good results is aeration-oxidation, or A/O. Apparatus costs between $100 and $200 and the kit comes with enough chemical reagents for just the first few tests. Because it ships as a hazardous material in larger quantities, you will need to source 25% phosphoric acid separately.
Finally, there is the method I have now adopted for all my sulfite testing: Vinmetrica. Dr. Richard Sportsman of Vinmetrica has adapted the Ripper method so that it does not require visually detecting a titration endpoint. Instead, a platinum electrode plugged into a proprietary meter measures an amperometric (electric current) endpoint. Chemical reagents are included when you buy the meter and replacements are readily available. The method is fast, easy, and accurate. I usually avoid endorsing specific products in my articles, but this is a rare case where I have done all of the methods and this meter stands well above the others. The SC100A meter for sulfite only, with enough supplies for about 50 tests, has a list price of $335. As noted throughout this article, knowing the pH of your wine is critical to choosing a target sulfite level. So if you do not already have a laboratory-quality pH meter, you might look to the SC300 kit instead. The meter can be used for both sulfite and pH, using different electrodes that are included. There are enough supplies for about 50 sulfite tests, plus supplies for about 30 titratable acidity (TA) tests and many pH tests. That kit lists for $599.
One final method for getting accurate test results is to pay a laboratory to do the analysis. In some areas, agricultural extension laboratories or university laboratories can do such testing. In major wine-producing regions, there are commercial laboratories that offer testing for sulfites and many other parameters. If you find a laboratory to do testing for you, talk with them about sampling methods, sample containers, and any shipping requirements. In my home area, Sonoma County, California, there are several commercial wine laboratories that offer fast, accurate results. For cost and convenience, though, I usually do my own sulfite testing.
As you progress in winemaking, I encourage you to learn about theory and practice in sulfite management. Research continues at academic institutions and this magazine will continue to publish emerging information on the subject. In the meanwhile, you now have a simple outline to get started: 50 ppm at crush. Another 50 ppm after all fermentation is complete. Analyze for free SO2 and adjust based on your target level from a sulfite calculator or pH chart. Every month, add 15 ppm, testing and adjusting every other month. Add 10 ppm more at bottling. Practical sulfite management for sound wine!