Protection from the Elements

We’ve all heard that great wines are made in the vineyard, and there is a lot of truth to that saying. It’s impossible to make high-quality wine if you’re starting with damaged or unripe fruit. Grape growing, harvesting, and fermentation are sort of the sexy part of wine production and thus these activities often come to mind first when we consider what winemaking is all about. Still, there are a group of activities that occur once the wine has been fermented that are also critical to quality wine production, especially for white wines. These processes and checks involve the chemical and microbial stability of wine. Once primary and malolactic fermentation are completed, chemical and microbial stability are the main concerns of the winemaker prior to final clarification, filtration, and packaging. I covered microbial stability in depth in the October-November 2022 issue, so this go-round we’re going to focus our discussion on heat and cold stability.
Before we dive into heat and cold stability perhaps it’s important to discuss what is at stake in terms of wine quality. Both heat and cold stability issues produce aesthetic defects in wine; they impact how the wine looks in the glass but not how the wine smells or tastes. Cold stability problems may slightly impact flavor by changing the acidity of the wine by a few tenths of a gram per liter — usually an imperceptible amount. There are some exceptions to this rule that we’ll discuss later. Heat stability problems don’t have any impact on the aroma or taste of wine. So, as a home winemaker are these stability issues important? The answer is literally in the eye of the beholder. Commercial winemakers are concerned about these defects because consumers and distributors may reject products with haze or sediment as unfamiliar and potentially faulty. The haze or sediment formed by heat instability looks the same as that formed by microbial instability (refermentation in bottle or microbial spoilage), so there is some potential quality concern if you’re a retailer trying to sell hazy wine. Consumers have also had limited experience with haze in wine, and a hazy wine may not be what they are hoping to post in their Instagram feed.
Still, in the craft beer movement there has been a sea change in acceptability of hazy products. Very hazy beers are even strongly desired in some styles like New England IPA. The natural wine movement has also shown that some consumers are willing to accept what used to be considered aesthetic flaws when the winemaker can explain how their limited intervention in the winemaking process has led to a product with fewer additions and “manipulations.” The rediscovery of ancient wine styles like orange wines have also influenced our notions of acceptability with regard to wine clarity. So, as a home winemaker the choice is yours, but understanding the causes and processing options around heat and cold stability will give you the knowledge to choose what is best for your wines. Let’s explore these instability issues in detail.
Heat (protein) Stability
Heat stability is also known as protein stability, because the levels of certain proteins in wine seem to be the main contributing factor in whether this particular instability may occur. Proteins in wine mostly come from the grape berries themselves, but may also be added when yeast autolysis occurs or when protein-based fining agents such as gelatin and egg whites are utilized in the winemaking process. Proteins are long polymers of amino acids. Each amino acid has a unique side chain, a branch of the molecule that is not part of the polymer backbone. These side chains contain chemical groups that can carry partial electrical charges, or may contain many carbon atoms or aromatic rings with no charge at all. The amino acid side chains and their placement in the protein backbone chain control their attraction or repulsion to other amino acids in the chain, as well as their hydrophilic or hydrophobic nature to the solution surrounding them. The interplay between these forces creates the three-dimensional structure of the protein.
The structure of a protein is instrumental in protein signaling and function, but also protects the hydrophobic parts of the amino acid side chains from the water and other ions in solution. Most proteins found in wine have an overall slightly positive charge because of the relatively low pH levels, and thus tend to repel other proteins in the solution.
The complicated three-dimensional structure of proteins are somewhat delicate, and protein structure may become unstable if solution temperature is increased, thus the reason why protein stability is also known as heat stability. Ethanol and pH changes can also impact protein stability, and some wines may throw a protein haze in bottle even without experiencing high temperatures. When proteins are no longer able to maintain their three-dimensional shape they tend to agglomerate together because of the partially charged and hydrophobic parts of their amino acid structure. As the disrupted proteins aggregate they become too large to be held in solution and they precipitate out of solution as a wispy, amorphous solid.
Heat stability is usually only a concern in white and rosé wine production where the haze and sediment formed from heat instability are more noticeable. Besides being darker and thus any haze is less likely to be noticeable, red wines contain tannin polymers extracted from skins, which are absent or at low levels in whites and rosés. Tannin polymers contain many hydroxide units in their structure that take on a slightly negative charge and thus attract the slightly positively charged proteins. This protein-tannin matrix, like agglomerated proteins, becomes large and precipitates from solution during the winemaking and aging process so there is little protein left to form a haze in aged red wines with appreciable tannin levels.
Heat Stability Testing
It is difficult to predict protein levels in wines. Different grape varieties contain differing levels of protein. Some varieties such as Grüner Veltliner are known to produce high levels of protein haze, while other varieties produce low levels. Vine nutrient status and cropping level also play a role in juice protein content, so it is usually necessary to test the protein stability of each wine lot each vintage.
The most common protein stability test is a heat test. A small sample of wine is sterile filtered, then heated to a particular temperature for a set period of time. The sample is then cooled and measured for turbidity to determine if proteins have denatured and caused a haze to develop. A common time and temperature regime is to heat the wine to 176 °F (80 °C) for two hours. Wine analysis laboratories use a temperature-controlled water bath, but a crock pot set to the high temperature setting is an inexpensive alternative. Accurate turbidity measurement requires a turbidity meter, but a home winemaker could also judge acceptable haze levels by sight. Large commercial producers shoot for haze levels below 1 NTU (nephelometric turbidity unit), which is brilliantly clear to my eye. Small producers are willing to accept higher haze levels, perhaps 2–5 NTUs, or simply gauge the haziness and acceptability by sight. As long as the product is not appreciably hazy following the heat test a winemaker may be satisfied that the wine is protein stable. It’s important to stress that the wine sample must be filtered clear prior to the heat test, otherwise it is impossible to judge whether haze observed after the heat test is coming from particles in the original sample or haze generated by denatured proteins.
Heat Stability Treatment
The most common treatment for wines with appreciable protein haze is fining with bentonite. Bentonite is a type of clay that is mined, kilned to remove impurities, and sold as a grainy powder. On a microscopic level bentonite consists of sheet-like particles with a large surface area. The sheets possess a slight negative charge, and thus attract the slightly positively charged proteins to their surface. Bentonite is insoluble in wine, eventually settling on the bottom of the storage vessel. When the clean wine is racked off the bentonite lees the proteins remain with the lees and are thus removed.
Unfortunately, bentonite is not selective for removing only protein, so over-fining with bentonite can actually strip aromas from wine. Because of this potential for over-fining it’s important to perform benchtop trials at varying bentonite addition rates, then perform a heat test on the trial samples to determine the optimum level of bentonite addition. In the U.S., bentonite addition rates have traditionally been displayed in units of pounds per thousand gallons (#/1Kgal). Most wines respond very well to addition rates of 0–4 #/1Kgal, but wines occasionally require up to 8–10 #/1Kgal to achieve stability. Chart 1 shows a typical bentonite fining trial result from a commercial laboratory. The control wine is slightly unstable, with a heat test NTU of 5.1, but the lowest addition at 1.0 #/1Kgal produces a heat stable wine. Increasing addition rates above 1.0 are unnecessary and unwarranted for this wine due to potential flavor stripping at high bentonite addition rates.
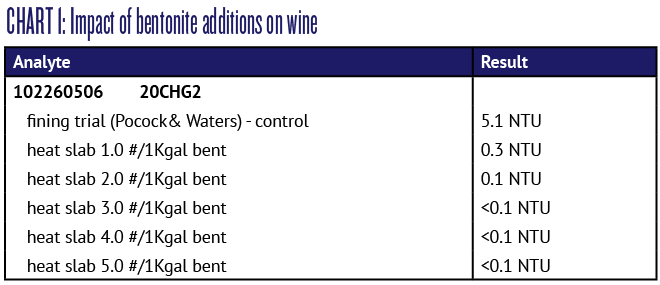
When performing a bentonite fining trial combined with the heat test, it should be stressed again that each sample must be filtered to clarity prior to performing the heat test. This means that all the bentonite added must be filtered out, otherwise the increasing rates of bentonite addition will cause increasing haziness in the finished sample.
Bentonite must be properly hydrated in hot water, never wine, to prevent clumping and maximize the surface area of the clay sheets for attraction of protein. I’ve found the best method is to slowly mix the bentonite into water while vigorously stirring — a stir plate and stir bar work well for small volumes. Some instructions from bentonite suppliers recommend allowing the bentonite to hydrate for 24 hours before addition. When adding the bentonite slurry to the wine the entire tank should be mixed or pumped over in a loop to ensure the bentonite has access to all the protein in the wine. The adsorption of proteins onto the bentonite surface is very fast, but bentonite settles relatively slowly, and may make a “fluffy,” uncompacted lees layer. Because bentonite is inert and not an energy source for microbial metabolism it may be left in the tank for a long period of time prior to racking with no deleterious impact. Thus some winemakers perform bentonite fining and traditional cold stabilization at the same time because they feel the bitartrate crystals help compact the bentonite lees, and there is no penalty for long contact of the wine with bentonite.
As a final thought on heat stability, some winemakers add a low amount of bentonite prophylactically during the juice settling stage after pressing, with the hope that little to no additional bentonite will be needed once fermentation is complete. These winemakers believe a juice addition of bentonite has less impact on wine aromatics than later additions when fermentation is complete.
Cold (bitartrate) Stability
Cold stability is also known as bitartrate stability (sometimes shortened to just tartrate stability) because this stability issue involves the formation and precipitation of potassium bitartrate crystals in wine. Like heat stability, bitartrate stability is primarily a visual defect. However, bitartrate crystallization and precipitation does impact the titratable acidity of wine by lowering the amount of bitartrate ions in solution, and may drive the wine pH higher or lower, depending on the pH prior to crystallization. These acidity impacts are usually minor, but may be a concern when wine pH is very high or extraordinarily large quantities of crystals are formed.
Bitartrate crystals often look like sea salt crystals, but may also appear as sandy clumps, and usually contain some of the same color as the wine. Savvy wine consumers don’t mind a few bitartrate crystals in their wine, often referring to crystals which form on red wine corks as wine diamonds. In white wines however, large commercial producers worry that some consumers may mistake the crystals for glass shards, or that consumers may simply be uncomfortable with solid particles floating around the bottom of their glass.
Potassium bitartrate has a slightly acidic, slightly salty taste and is used in baking, labeled as cream of tartar, to stabilize meringues and whipped cream. Bitartrate crystal formation in wine is a complex chemical and physical phenomenon with many factors impacting crystal growth and precipitation. The process begins with a super-saturated solution of bitartrate ions and potassium ions. Both potassium and bitartrate ions are found in grape berries. Bitartrate ions are one of the forms of tartaric acid, which is often the most plentiful acid in grapes. Tartaric acid exists in different forms in solution depending on the pH. At typical wine pH the majority of the tartaric acid is in the bitartrate (HT– form). Potassium ions are found in berry pulp but are also quite prevalent in grape skins, so wines with skin contact often have higher levels of potassium.
Supersaturation in liquid solutions occur when there is more of a solid solute in solution than the solubility equilibrium for the solute. Supersaturation commonly occurs due to temperature change. The process of making rock candy is an example of this phenomena. Sugar, the solid solute, is supersaturated in a water solution by dissolving the sugar when the water is boiling. When the water cools the sugar is supersatured, and placing a stick into the solution will cause the sugar to crystallize and precipitate onto the surface of the stick. Besides temperature effects on solubility, the addition of other liquid solvents to a solution may also impact solubility. In wines potassium bitartrate solubility equilibrium is lowered with increasing ethanol content, thus fermentation increases the potential for supersaturation of potassium bitartrate.
The next steps in potassium bitartrate precipitation are growth of a crystal structure. In order for the crystal to be formed it must have a nucleation site that lowers the energy needed to begin crystal formation. Small solid particles in solution (like grape solids) are good nucleation sites, as well as perturbations in solid surfaces, thus crystals often form on the surface of tanks, barrels, bottles, or corks. Once a crystal begins formation at a nucleation site the rate of crystal growth must be greater than the rate of crystal deconstruction in order for crystal growth to occur. To grow the crystal structure large enough to be seen there must be availability of more potassium bitartrate molecules in the vicinity of the crystal edge, and the dissolved potassium bitartrate must connect onto the crystal surface in the exact geometry that continues the crystalline structure of the growing crystal.
Wine is a complex chemical solution, and potassium bitartrate crystallization may be impacted by interactions with other molecules and macromolecules. Figure 1 displays a detail of the chemistry involved in the formation of potassium bitartrate from potassium and bitartrate ions (on the left side of the image), as well as the steps in crystal growth and eventual precipitation of solid potassium bitartrate crystals (on the right side of the image).
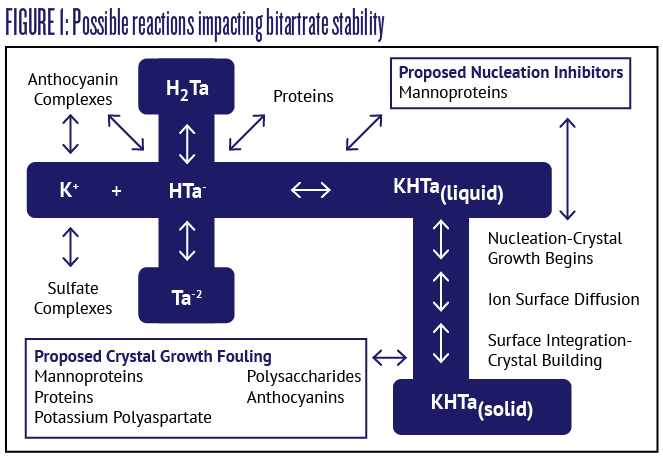
This figure also shows some of the interactions that occur in solution between ionic species and other molecules, and some of the molecules and macromolecules thought to inhibit crystal formation or growth. These interactions make it difficult to predict bitartrate stability in wine simply by measuring the quantity of potassium and bitartrate ions in solution. Complexing of ionic species with anthocyanins is thought to be the reason why bitartrate precipitation occurs more slowly in red wines. Another example of these interactions is crystal growth fouling and nucleation inhibition caused by proteins and mannoproteins from yeast autolysis, which is thought to be the reason why wines aged on yeast lees have enhanced bitartrate stability. Scientists have utilized these inhibitory interactions to propose new additives for promoting bitartrate stability, which will be discussed next.
Achieving Bitartrate Stability
Given the number of factors involved in potassium bitartrate precipitation there are also several different methods for achieving tartrate stability (see Figure 2). Starting at the bottom left of the figure is the classic method for achieving bitartrate stability, the formation and removal of crystals prior to bottling. Because potassium bitartrate solubility is strongly influenced by temperature, if wine is stored for a period of time at a temperature well below its expected future storage temperature prior to consumption, then the amount of bitartrate crystals formed in the storage vessel should deplete the potassium bitartrate concentration sufficiently to ensure more crystals will not precipitate in the future. The wine is then removed from the bitartrate crystals in the storage vessel by racking or filtering the wine while it is still cold.
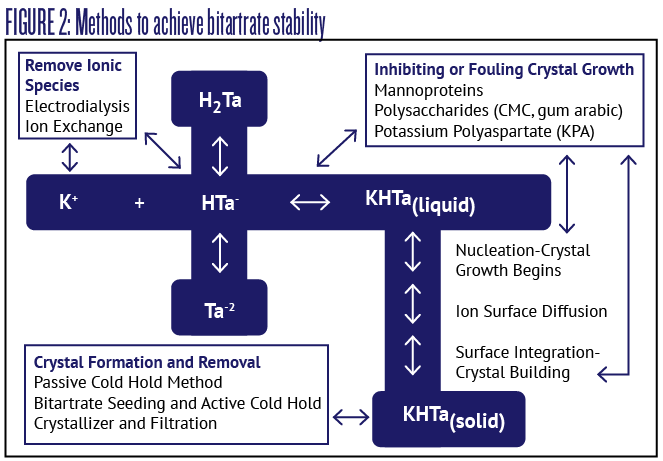
To achieve bitartrate stability commercial winemakers often shoot for a temperature goal of around 26–28 °F (-3 to -2 °C) for one to six weeks. Some winemakers speed up the process by lowering the temperature and/or adding potassium bitartrate as a crystal seeding agent while mixing. Standard rates of seeding addition are quite high: Somewhere between 1–4 g/L. In cold climates tank cooling may be assisted by outside winter temperatures, but in warm climates the energy cost for cooling large tanks is quite high. Commercial technologies are often used in these areas; such as crystallizers that quickly promote crystal growth and then the crystals are removed.
Speaking of commercial technologies, large commercial producers also have the option of using electrodialysis or ion exchange to remove the ionic species responsible for crystal formation (upper left box in Figure 2). This has the benefit of a limited waste stream, low sensory impact on the treated wine, and it can be performed at cellar temperatures — eliminating the huge energy costs required with the traditional method.
When potassium bitartrate crystals are removed from solution in traditional cold stabilization there is an impact on the pH and titratable acidity (TA) of the wine. For wines below pH 3.7, usually the case for white and rosé wines, the pH shifts lower by 0.1–0.2 pH units and the TA may drop 1–2 g/L. This generally does not have a large impact on taste or microbial stability. If the pH is greater than 3.7, however, the pH change will actually shift up, potentially leading to microbial concerns, while the TA drops, perhaps impacting the perception of tartness in the wine. For this reason, high-pH wines may be treated with tartaric acid additions prior to cold stabilization in order to maintain adequate pH and acidity after the cold hold process.
As mentioned earlier, several winemaking additives have been developed and approved for use in bitartrate stabilization. These additives either inhibit crystal formation, or cause fouling of the crystal surface to limit their size, thus the crystals don’t become large enough to precipitate (see box in the upper right of Figure 2). Like electrodialysis, these additives have the benefit of providing bitartrate stability without the time and energy cost involved in the traditional cold hold method. These stability enhancers also will not impact the pH and titratable acidity of the wine.
The polysaccharide harvested from acacia trees known as gum arabic has a long track record as an additive to help promote colloidal stability, and in some cases bitartrate stability when used with metatartaric acid. However, the bitartrate stability enhancement of gum arabic does not appear to work for all wines, and metatartaric acid provides only temporary stability, so additive suppliers began looking for other alternatives. Mannoproteins derived from yeast cells and carboxymethyl cellulose (CMC), a polysaccharide derived from plant fiber, are two more recent alternatives for bitartrate stability (approved for use in 2011 and 2012). CMC is probably one of the most common bitartrate stability additives in use in commercial production, and home winemakers may find both mannoprotein and CMC additives online. CMC has the advantage of being more shelf-stable than mannoprotein once opened, but some winemakers believe mannoproteins are more natural, since they are derived from yeast. CMC is not recommended for red wines, and mannoprotein application in rosé and red wines is also tricky, with complicated instructions for correct dosing, so suppliers have kept looking for new additives that can be utilized in all wine types.
A new bitartrate stabilizer with universal application is potassium polyaspartic acid (KPA). KPA is a polymer of aspartic acid, an amino acid common in plants and animals. KPA, like CMC, is long-lasting in wine, thus providing long-term bitartrate stability, but has the added benefit of working well with rosé and red wines. KPA was approved for use in commercial wines in 2020. I haven’t seen this additive in home winemaking shops yet, but as its use expands I expect it to become more readily available.
All of the bitartrate stabilization additives mentioned should be added a few days before final filtration and bottling if sterile filtration is desired. An important precaution is that wines must be free of haze and protein- and color-stable prior to their addition, otherwise the bitartrate stabilization agents may interact with the unstable components in the wine to produce a sediment.
Testing for Bitartrate stability
The multifaceted phenomena of bitartrate crystal formation makes testing for bitartrate stability complicated since both potassium bitartrate formation and crystallization kinetics are involved. One of the most common methods of determining stability, however, is quite simple and is known as the cold hold test. As a first look at bitartrate stability the Australian Wine Research Institute (AWRI) recommends a cold hold test for 3 days at 25 °F (-4 °C). This temperature is unfortunately colder than the common refrigerator temperature setting (~40 °F/4 °C), but warmer than the common freezer setting ~0 °F (-18 °C). The danger of using a refrigerator temperature setting for the cold hold test, even if extending the hold time to a week or longer, is that stability will be overestimated and bitartrate crystals may still form as the wine ages. The danger of using the freezer test is that the freezing phase change is a very aggressive test and will therefore underestimate the real-world stability of the wine. Many consumers often hold white wines in the refrigerator for a few days or weeks prior to consumption, but most don’t freeze wine prior to consumption unless they forget a quick-chilling bottle in the freezer!
So, perhaps a mini-fridge set to a very cold setting, or a long refrigerator test may be the best method of checking stability for the home winemaker. Large commercial producers need stability answers more quickly and precisely, so they utilize a special test apparatus that measures the conductivity of a small sample. The testing cell holds the wine sample at a constant low temperature, automatically adds potassium bitartrate as a seeding agent, and then continuously stirs the sample while measuring the change in conductivity over time. As potassium and bitartrate ions crystallize and precipitate out of the solution the conductivity of the sample will drop, so a large percentage change in conductivity indicates the sample is bitartrate unstable.
Cold and Heat Stability Summary
Methods for producing protein- and bitartrate-stable wines may initially seem complicated, but most winemakers develop a processing strategy and testing methodology that works for them over time. Their strategy is developed using the tools they have on hand and matching that to the wine styles they produce. If you’re unhappy with sediment in your finished wines your first job is to understand where the solids are coming from. Once you know the source you can try tweaking your winemaking with some of the ideas or products suggested until you also find a process, additive, and analysis protocol that works
for you.





