Sulfur Dioxide: Fact and Myth

Over the last 40 years, I have published a handful of articles and taught hundreds of classes about sulfur dioxide in wine. Beyond the basics, there exists a raft of common wisdom that is simply untrue.
Through this article I will draw on my own research over the last four decades as well as other important literature that has somehow failed to make its way into the mainstream enological academia consciousness, much less its disciples in commercial winemaking. What you’ll find, is that there are a lot of myths about sulfur dioxide that are widely believed. You can count on my viewpoint to be controversial.
Before I delve into this juicy subject, let’s review the basics.
What is SO2?
Please stop calling it “sulfur.” The chemistry of this element is complicated enough without confusing its four nearly unrelated branches: The sulfites, the sulfates, the sulfides, and elemental sulfur.
Sulfur dioxide (SO2) is a noxious gas produced by the burning of elemental sulfur. In its bound form, it is also produced in small amounts by yeast during fermentation.
Myth #1: Sulfur dioxide has been used since the early days of winemaking to protect oak barrels from microbial spoilage.
It’s use in wine is a recent development. According to ancient wine scholar William Younger’s masterful 1966 book Gods, Men and Wine the Romans used “blue smoke” as a pesticide in orchards, in magic, and in a primitive form of gunpowder, but didn’t like its effects on wine. There is no evidence that they or any prior winemaking culture used it in winemaking for the first seven millennia of recorded winemaking and the Roman Latin language records are quite thorough and readable, covering over half of Younger’s impeccably researched 564-page tome.
The earliest recorded use was by a Franconian monk named Martin Bayr in 1449. Inexpert use in the coming years led to its abuse being curtailed to only the sanitation of used casks in the Holy Roman Empire Wine Purity Act of 1498, according to sulfite-free winemaker Paul Frey.
Today, sulfite use is so prevalent that most winemakers, including many world-famous enologists, are under the false impression that sulfites are essential to winemaking.
Myth #2: Wines require sulfite to avoid spoilage.
According to Dr. Andrew Waterhouse, former Chairman of the Dept. of Viticulture and Enology at UC-Davis, “Without sulfites, wine is extremely perishable and should be refrigerated for its entire 1-year life cycle between harvest and then from the winery to consumer.”1
So how come my sulfite-free WineSmith Roman Reserve wines, nowhere touted as sulfite-free in marketing, are still my best-selling, most highly priced wines after 5–8 years in neutral cooperage?
Admittedly, it took me 40 years to acquire the knowledge to make such wines and I am not encouraging readers to jump into this challenging undertaking, so let’s move on to modern conventional winemaking.
I will also admit that I have no idea how to make sulfite-free white wines. Frey Vineyards in Redwood Valley, California, does it quite successfully by completely eliminating oxygen throughout the winemaking process as brewers do, resulting in modern, conventional, fresh whites. You can’t do this at home.
The Georgians do it by making amber wines with six months of skin contact, essentially alcoholic teas, which age for decades into complex, rich, dense wines that bear no resemblance to modern whites. This will work if you’re into this style and have the time to wait.
Free SO2 in White Wines
SO2 gas can be purchased in canisters in liquefied form under pressure and dissolved in water up to 9% (commercially available as 6%, which is safer) or combined with potassium hydroxide (KOH) to form K2S2O5, a white powder called potassium metabisulfite (KMBS). When any of these are added to wine, they distribute themselves (collectively known as sulfites) into equilibrium according to pH. The free SO2 equilibrium looks like this:
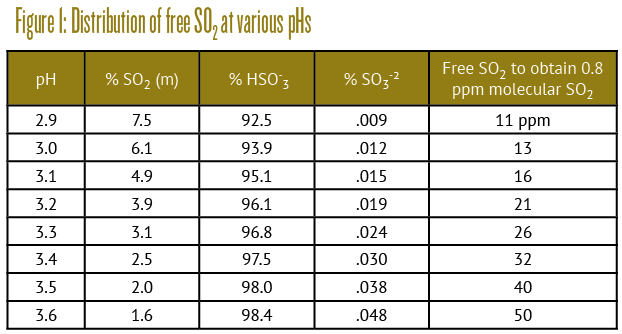
Note that the leftmost species has no charge and thus is quite volatile. It’s the only one you can smell. No, it doesn’t smell like rotten eggs — that’s H2S, an unrelated sulfur compound. It hurts to smell, rather like a freshly struck match. It’s only present in tiny amounts, but it’s the species that is toxic to microorganisms because it can easily penetrate cell membranes due to its neutral charge, thus pickling the cell in a similar manner to vinegar or pool chlorine. Because it also has an unpleasant smell, your job is to regulate its concentration at the pH of your wine, using the table in Figure 1.
You simply multiply your free SO2 determination by the stated percentage (times 100 to express it as a fraction) and shoot for 0.8% ppm SO2 (m). This is usually expressed as mg/L, which is effectively the same thing as ppm in these small concentrations. Note the ten-fold variation in this percentage over the pH range of wine.
The bisulfite form (HSO3–) doesn’t fight microbes. It inhibits a specific browning enzyme called polyphenol oxidase (PPO) and not much else. It does combine with an oxidation product of ethanol called acetaldehyde (the smell of stale apples and fino Sherry) to form the acetaldehyde-bisulfite complex (ABC), which is in slow equilibrium with the free SO2 (FSO2).
The right-most species is called sulfite (SO32-) and is present in extremely low concentration and thus doesn’t do much. It can react with oxygen: 1⁄2 O2 + SO32-, but this reaction is very slow, occurring over weeks and months, which brings us to:
Myth #3: FSO2 is a potent anti-oxidant that scavenges oxygen and protects wines from oxidation in racking and bottling.
In fact, FSO2 will not prevent aromatic freshness from turning to staleness nor prevent browning, and over the course of a couple months post-bottling will itself oxidize in about a 4-to-1 ratio, so that if your bottling dissolved oxygen is 4 ppm, typical of a hand filler set-up, your 15 ppm of FSO2 will disappear completely. So yes, it reacts, but at a sub-optimum rate.
Measurement

FSO2 analysis must be done onsite. Its level seriously deteriorates on the way to the lab you send it to for analysis. For home winemakers, there are three methods for measuring SO2: Titrets, iodometric (Ripper) titration, and aeration/oxidation. Let’s take a closer look at these:
1. CHEMetrics Titrets run about $1 per analysis. I hate them. They are tricky to use and are laughably imprecise. They come in various levels, so you have to know the range you’re in before you start. They will not guide your wine caretaking very well. Weigh your savings against potentially needing to chuck your spoiled wine down the drain.
If you’re serious about winemaking, save up for a real analysis. This will probably involve your learning to titrate. This involves acquiring a stand supporting a burette (a graduated cylinder with a valve on the bottom) that you fill with a reactive solution of known strength. You place a known quantity of wine in a beaker or flask along with a dye that will indicate when reaction is complete. You dribble the reagent from the burette until the dye changes color, at which point you stop, read how much titrant you used, and calculate your FSO2 from a formula.
2. Iodometric titration has several forms, all involving iodine titration. (Titrets are iodometric titration, too, but an unfortunately oversimplified version.) Until recently, the indicator dye was potato starch, which turns blue when excess iodine is present. This works fine for white wines but with reds, the endpoint is hard to see and phenolics present substantial interference. Today we have Vinmetrica SC-300, which overcomes these problems by substituting amperometry for potato starch. It includes a pH meter and titratable acidity (TA) capability. The whole setup with the stand is about $750 and is a boon to the home winemaker.
3. Aeration / oxidation: This is the most popular method for small wineries. It involves drawing air through an acidified sample of wine and trapping the SO2 with hydrogen peroxide, producing sulfuric acid (H2SO4), which can be titrated. The vacuum can be drawn with a small, inexpensive pump on a 10-minute timer. Such a setup costs about the same as a Ripper setup, but you don’t get the pH meter.
Both of these have alternative analysis for total SO2 (TSO2), from which we can calculate the bound by subtraction. I won’t go into the details here, because in most instances, this isn’t very useful information, so they are not routinely run unless your wine is unaccountably gobbling up SO2 additions. Standard winemaking results in TSO2 not much over 100 ppm, and 200 ppm is where a soapy, oniony flavor begins to appear in the finish.
Health Issues
I’ll estimate that 10% of the wine-drinking public are concerned that they have an allergy to sulfites. Since the human body produces about one gram of sulfites per day, or about ten times the amount in a typical bottle of wine, this is obviously impossible. The anaphylactic shock diners have experienced when filling their plate with lettuce containing pure crystals of sodium bisulfite (to prevent browning) and then dowsing it with low-pH vinegar is not an allergic reaction. It’s SO2 gas exploding from the plate to your nose and precipitating acid rain in your lungs.
I disagree with Paul Frey that sulfite-free wines are necessarily good for your health. We do, however, agree that modern medicine is pretty clueless in this area.
That doesn’t mean that sulfite-free wines aren’t interesting. I make them myself and consider them my best wines. But sulfite-free wines tend to contain ten times the level of biogenic amines, a major source of red wine allergy with lovely names like putrescene and cadaverine, the smells of dead animals.
Nevertheless, they can be the most profound and long-aging wines you’ll ever encounter. But they’re a high risk/high reward delicacy like Eppoises, half-shell oysters, or fugu.
Sulfite-free winemaking is a viable enterprise for the master winemaker who is not risk-adverse.
Myth #4: Even if no sulfur dioxide is added to wine, fermenting yeasts will produce SO2 from the naturally occurring inorganic sulfates in all grape juices. Thus, it is impossible for any wine to be completely free of sulfur dioxide.
Not really. The reaction of diphenols and oxygen produces a byproduct of hydrogen peroxide (H2O2), which instantly reacts with any sulfites, be they free or bound, converting them to sulfate (SO42-), which lacks sulfite properties. This happens when young red wine receives any oxygen, as in racking, barreling down, or during enological oxygenation, so a great many un-sulfited reds have no detectable sulfites, as with my WineSmith Roman Reserves.
Thresholds
In 1984, I wrote an article titled “SO2: The Limits of Our Understanding,” which explored the dual questions, “How much is enough?” and “How much is too much?” Besides the health issues addressed earlier, the latter involves sensory detection.
In 1982, Patricia Howe and I while in Rosemarie Pangborn’s lab at UC-Davis, looked into sensory thresholds. Incredibly, our literature search revealed that the only sensory study ever done on SO2 in wine was a 1955 survey of 56 compounds by Hinreiner and Berg. Because of flawed methodology, they reported the sensory detection threshold at an astounding 120 ppm!
Pat and I developed an ascending dual quadrangle test that was far more sensitive — so much so that we never did find the actual threshold, defined as the point where 50% of subjects can find the spiked sample. All I can tell you is that 24 out of 36 subjects were able to pick out 0.45 mg/L, nearly half the recommended storage maintenance level of 0.8 mg/L. This doesn’t mean they disliked the spiked sample, nor did they recognize what was causing the difference.
We were able to gauge individual thresholds with 94% confidence and thus were able to find no unusually sensitive individuals, no bifurcated threshold groupings.
Our study also measured equilibrium headspace compared to ideal solution behavior and found a close match to Henry’s Law predictions. Remember, this is all white wine.
Since our senior project was never published in a peer-reviewed journal, Berg’s finding remains today, as far as I know, the only published research on SO2, attesting to the thin coverage of basic enological research in the literature. Please say it isn’t so.
SO2 in Red Wines

Here’s where it all comes off the rails.
Pat continued this work in elaborate studies for her doctorate at Cornell, culminating in an important yet as yet little-known thesis. The startling revelations of this work include:
• FSO2 ain’t free. When you directly measure the SO2 in the headspace above a red wine, you find little or none. What we are measuring with standard Ripper methods or aeration-oxidation excludes those bound by acetaldehyde because it is in slow equilibrium with the free. But the portion bound to pigments, especially young monomeric anthocyanins, is in rapid equilibrium with the true free, and due to mass action, is drawn in and included as part of the so-called “FSO2.” FSO2 ain’t free.
• Forget about molecular SO2 calculations. Free SO2 doesn’t actually exist at all in young red wines, as it is all bound to anthocyanins, and is well below ideal concentrations even in older reds.
So what does protect red wines from spoilage? Two answers.
The first is competition from beneficial organisms. Prior to the turn of the millennium, all wine microbiology was based on agar plating. We now know that the vast majority of wine organisms can’t be plated.
Dr. Charles Edwards and Washington State University have pioneered the use of PCR (as in COVID DNA testing) to unlock the mysteries of wine microbiome. The vast majority of wine organisms are either benign or beneficial, imparting the treasured bottle bouquet whose profundity lured many of us into the biz in the first place, and to out-compete Brettanomyces for nutrients. This microbiome does not flourish in the presence of draconian cellar practices such as excessive SO2.
What does flourish? You guessed it — Brettanomyces. It’s an opportunistic pathogen, a hospital disease caused by excessive sanitation. Since it employs a whole suite of clever practices to hide out from SO2, what the preservative mainly accomplishes is to kill off beneficial microbiome, leaving a clear path for Brett. Although petri dish plating of sulfited wines does show diminished numbers, Dr. Edwards discovered that the preservative renders it “viable — nonculturable.” Enology’s funniest joke.
That joke was on me. My Master’s thesis was on SO2 levels necessary to kill Brettanomyces. I isolated 58 cultures from California wines and tested the level necessary to kill as defined by a one-million-fold decrease in plateable colonies in 24 hours. Since SO2 makes Brett viable-nonculturable, turns out it was a total waste of time.
The second protection of red wines from Acetobacter spoilage is the wine’s ability to absorb oxygen, or “O2 appetite.” Aline Lonvaud-Funel at University of Bordeaux discovered in 2000 that pigment-bound SO2 is ineffective against vinegar bacteria. The real take-home message is that your reds will take care of themselves if made from sound, properly ripe (but not over-ripe) grapes so that they have a healthy oxygen-consuming reactivity. One sign of high reductive potential is the production of small amounts of H2S.
Paradoxically, SO2 disrupts red wine’s natural anti-oxidative capability, short-circuiting oxidative polymerization, in our measurements, by 92%, thus inhibiting the wine’s natural immune system by over an order of magnitude. Thus, SO2 acts, not as a reducing agent, but as an anti-oxidation suppressant. Not good.
So what, if anything, is the function of SO2 in red wine? My opinion of the desirable pH zone for reds is between 3.70 for light reds and 3.85 for big, long-aging Cabernets and such. In this zone, conventional wisdom is to maintain 20 to 30 ppm “free” to scavenge aldehyde without reference to pH, thus avoiding oxidation and browning. FSO2 has almost no beneficial anti-microbial effect, so molecular SO2 levels are irrelevant.
I should stress that there is no middle ground. If you have any FSO2 at all, you will experience phenolic O2 appetite suppression. You must intentionally choose either an all-in no-sulfites regimen (only for experienced hands) or stick with the conventional 20–30 ppm.
You may be wondering why all this is news. Enology, like all modern science, is politics. We tried to publish the thresholds work along with my Brettanomyces study in 1986, but politics intervened. The American Society for Enology and Viticulture seemed to think that if we kept our heads low, sulfite labeling could be prevented. Yeah, right — that certainly worked well. This is how academic publication politics works.
I know that my views are controversial. While conventional enology recommends a safe path to good wine, I point to a methodology for greatness. This is where home winemakers can outshine commercial wines. As long as you’re not using winemaking to feed your family or your community, you can afford to take more risk and embrace advanced techniques outside the box that have solid scientific foundations and can lead to greatness seldom achieved in the commercial game.
References:
1 https://blog.wblakegray.com/2011/05/sulfites-in-organic-wine-update.html
* The numerous studies and papers referenced in this article are too long to list in full here. They may all be found through the Dropbox link at http://postmodernwinemaking.com/sulfur-dioxide-revisited.