Tannin Chemistry in Wine
It all starts with phenol. The tannins in wine make up one of the subgroups in a larger chemical family known as polyphenols. Those, in turn, are made up of phenol-derived molecules and additional carbon, hydrogen and oxygen atoms.

While a winemaker does not need to know the chemistry of polyphenols and tannins to make good wine, some basic knowledge can help drive winemaking decisions that may improve the outcome of any given vintage. So, if you like a bit of technical illumination along with your homemade wine, follow us here into the magical world of these complex and fascinating chemical compounds.
Benzene and Phenol
Phenol can be viewed in terms of its derivation from the benzene ring. The chemistry and reactions of benzene, formula C6H6, were well understood in the 19th Century. Its structure, however, eluded researchers of the time until August Kekulé had a dream of a snake biting its own tail. That gave him the idea that perhaps benzene was a ring of six carbon atoms, each with one attached hydrogen atom. At the time, the numbers added up and the properties made sense. Subsequent investigations showed that benzene was indeed a ring, with every other carbon-to-carbon bond a single bond, alternating with double bonds. (Linus Pauling later refined the every-other-bond idea as quantum mechanical “resonance”, but that’s another story.)
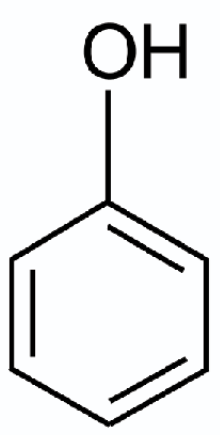
As with all large organic (carbon-containing) molecules, benzene is often represented as a line drawing or “skeletal formula.” In a skeletal formula, the bonds between atoms are represented by a single line, or two or more parallel lines, corresponding to single, double or higher bonds. Individual atoms are given as their chemical symbol, the one or two-letter code for the elements as seen in most periodic tables. Because carbon atoms are the focus of organic chemistry, they are not written out in a skeletal formula; they are implied at any intersection of chemical bonds and at the end of any depicted bond. Likewise, because hydrogen atoms are plentiful, they — and the bonds to them — are generally not written out unless doing so makes the diagram more helpful. They are simply assumed to be bound to any unfilled chemical bond. This way of depicting molecules is also attributed to August Kekulé.
The skeletal formula of benzene is a hexagon (a six-sided polygon) with the sides of the polygon being represented by alternating single and double lines. The single and double lines represent single and double bonds and the six “points” on the hexagon, where the bonds meet, are where the implied carbon atoms would be. Each point on the hexagon has three bonds extending from it (one single bond and one double bond), yet we know that carbon forms four bonds. So, the fourth bond is filled by the assumed hydrogens and the bond leading to it. These point away from the ring, when shown.
Some modern skeletal formulae depicting benzene, or any molecule that includes a six-sided carbon ring, show the hexagon with a circle (or sometimes a dotted line circle) inside. This reflects the fact that alternating single and double bonds in a ring are fluid.
Phenol is a benzene ring with one of the hydrogen atoms replaced by a hydroxyl group. A hydroxyl group is composed of an oxygen and a hydrogen atom, written as –OH in a skeletal formula.
That addition of an oxygen atom is critical to the chemical behavior of phenol and the compounds built up from it. If you are already familiar with wine chemistry, you will recognize that a hydroxyl group is also a marker for alcohols. Most prominent, of course, is ethanol: C2H5OH. Wine may also contain traces of methanol (CH3OH) and higher alcohols, all with a hydroxyl group attached. While bearing that structural similarity to alcohols, phenol behaves differently due to the benzene ring to which the hydroxyl is attached.
Phenol is a polar molecule that is somewhat soluble in water and slightly acidic. Phenol has a variety of commercial applications, including being a building block of polycarbonate plastics, nylon and many pharmaceutical products. Pure phenol causes severe chemical burns and is a neurotoxin. (Obviously, the tannins and other polyphenols in wine do not exhibit these properties.)
In grapevines (and other plants), phenol is synthesized and combined into multiple-ring compounds to produce the polyphenolics. In winemaking books, you may have read about not just tannins, but also flavonoids, anthocyanins and other members of this diverse group.
No discussion of tannins can be complete without touching on the relationships and reactions with some of those related compounds.
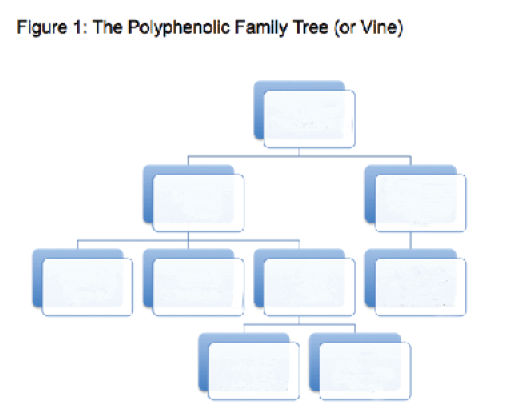
Figure 1 presents a simplified table of family relationships to help keep track of the players as we go through them here. Our review of tannins will include what they are, their role in winemaking, and their effects in wine tasting.
From Figure 1 we can see that “tannins” as a group make up one of the subsets of flavonoid compounds, which are in turn a subset of the total family of polyphenolics. Total polyphenolics in wine range from about 100 to 1,000 mg/L (ppm) in white wine and about 300 to 3,000 mg/L in reds. While that total, topping out at only 0.3%, may seem small, it is the next largest contribution to wine makeup after water, alcohol, acids and (possibly) sugar. The polyphenolics contribute to taste, mouthfeel and color. They also have anti-oxidant properties.
Tannins in Plants
Tannins are bitter and astringent. These characteristics likely contribute to the role of these compounds in the plants that harbor them.
Tannins are widely distributed throughout the plant kingdom. They are common (but not ubiquitous) in both Gymnosperms (conifers, cycads and gingkoes) and Angiosperms, flowering plants that include monocots (which include grasses and orchids) and dicots (which include most trees, including oak . . . and grapes). Tannins are distributed in different tissues of grapevines, with about 58.5% in the seeds, 21% in the stems, 16.5% in the leaves, and 4% in the skins. Tannins are generally sequestered in vacuoles (membrane bound “sacs” within the cell), most likely to avoid interference with cellular activity. Their unpleasant taste probably helps protect against being eaten by herbivores while the crop is maturing. As grapes ripen, the tannins in the skins also are said to “ripen,” becoming rounder, softer and less aggressive over time.
Seed tannins remain harsh. As foraging animals eat the grapes, they will be unlikely to chew the seeds. New grape vines earn an opportunity to grow. Seed tannins also have allelopathic properties (they suppress the growth of competing plants) and anti-bacterial properties.
The Flavor and the Feel
Bitterness is one of the four flavors for which we have specific taste buds; the others are sour, sweet, and salty (plus maybe umami, or “savoriness”, but that rarely comes up with wine). Astringency, on the other hand, is a physical effect. It is a puckery, drying sensation, such as found in tea when the tea bag has been left too long in the cup. That tannins should produce astringency comes as no surprise: it is at the root of their very existence. Tannins are so named because they are used in the tanning of leather — the word derives from an old German term for oak or fir trees. When raw animal skins are soaked in a bath containing tannins (originally from tree bark), the tannins react with proteins in the skin, causing them to become less water soluble and more resistant to decay: leather. The tannins in your red wine bind to salivary proteins in your mouth, drying and irritating the tissues. The same reactions make it possible to fine out excess tannins in wine with proteins, such as egg whites or gelatin.
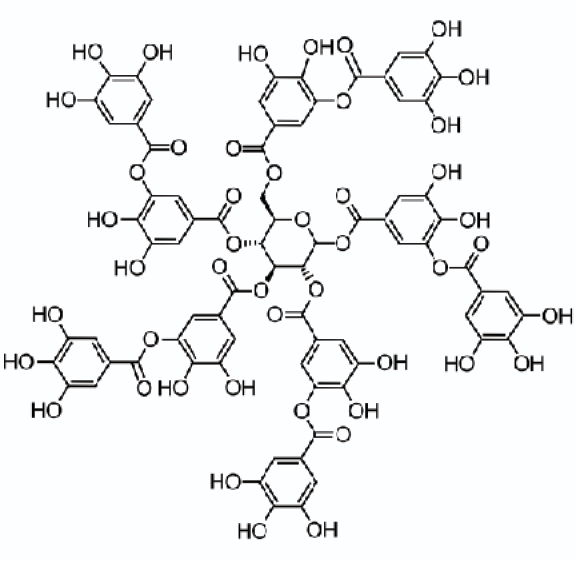
Tannic Acid
So far, we have not identified the chemical structure of “tannin.” That is because it is not a single, identifiable chemical compound like ethanol or benzene. Rather, “tannin” refers to a family of plant polyphenols that are (or could be) used to achieve the tanning of leather.
The chemical literature will most often cite tannic acid as “tannin”, but even that is not very precise. Tannic acid is considered as a reference compound for some purposes in discussing tannins, but its formula is non-specific, often given as C75H52O46 with a molecular weight of about 1,700.
Purified tannic acid may be purchased and even added to wine, but it is not the same compound as the grape or oak tannins native to wine production. In choosing a way to characterize tannins, chemists have also looked to their sub-groups, especially gallic acid.
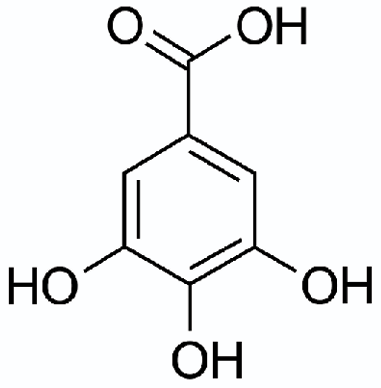
Gallic Acid
In particular, tannin molecules may be viewed as built up gallic acid molecules. Gallic acid — also called 3,4,5-trihydroxybenzoic acid — is a phenolic ring that has three hydroxyl groups, rather than just one (as with phenol itself). Gallic acid is abundant in tea leaves, witchhazel, hops and oak bark. It is a component of iron gall ink — a purple-black ink used for centuries in Europe. It is an anti-oxidant and has also shown some anti-cancer properties. Analytical methods have been developed to determine the total polyphenolic content in wine in terms of “gallic acid equivalents” or GAE. That number puts all tannins — with molecular weights from less than 1,000 to over 4,000 — on an equal basis for discussion (even if we do not identify exactly what each of them is). Tannic acid is generally depicted as containing two to twelve gallic acid-derived units and its molecular weight is about ten times that of gallic acid itself.
Grape tannins may be reported in gallic acid equivalents, but their structure can better be described as built up from catechin, epicatechin and related compounds. These molecules contain two phenolic rings, each with two hydroxyl groups, connected by a non-benzene ring. As grapes mature, these relatively smaller units combine to form polymers — usually between two and eight units in size. As the polymer size goes up, so does the molecular weight. The higher molecular weight tannins that form are generally less astringent and less harsh to the taste. It is largely this combining of tannin precursors and small tannins into larger tannin molecules that accounts for descriptions such as “less green” and “more supple” in the tannin profile of well-ripened fruit.
Tannin Extraction
Once the tannins develop in the fruit, they are ready for the winemaker to get them into the wine. Tannins can be dissolved in water and alcohol at different rates, so extraction of tannins continues as long as juice or wine is in contact with skins, seeds, stems or pulp. White grapes are lower in tannins than red grapes to begin with and juice is generally separated from other grape components by prompt pressing.
Some winemakers prefer to crush white grapes, freeing some of the tannins, and allow some period of skin contact to extend tannin extraction. Even so, as noted earlier, finished white wines are much lower in tannins than red wines.
Tannins are more easily extracted in high pH solutions. However, as pH has many important consequences in winemaking, winemakers never attempt to alter wine pH simply to enhance or retard tannin extraction.
In reds, extraction begins with crushing, releasing tannins from the skins (pulp and juice tannin content is quite low). In the same crushing operation, related polyphenolics, anthocyanins and flavonols, also begin to be released. Anthocyanins are red and blue pigments that account for most of the color in red grapes and are concentrated in the skins. Flavonols, including the compound quercetin, are yellow pigments with a bitter flavor.
Both groups are more extractable in water (or juice) than the tannins. During cold soaking, anthocyanin concentration in the juice goes up quickly, producing a brightly colored red solution. Once fermentation begins, the anthocyanins begin to combine with tannins to form other, less brightly colored, red pigments. Some of these reaction products precipitate and fall out in the lees, reducing wine color. To minimize this effect, some winemakers add enological tannins as “sacrificial” tannins to react and fall out, leaving the native grape tannins to become part of the finished wine. Enological tannins are extracted from a variety of sources including grape seeds, chestnut and oak wood, quebracho wood and oak gallnuts. (Several of these sources were used to produce tannins for leather tanning long before they were selected as enological addition products.)
Seed tannins, as noted earlier, are considered harsh. Care is taken to avoid crushing the seeds to minimize their extraction. For some delicate red wines, such as Pinot Noir, a special rack-and-return technique called délestage is employed where seeds are removed from the wine each time it is pumped back onto the cap. Stem tannins are often considered “green” tasting, but the same winemakers who practice délestage to avoid seed tannins may return some of the stems (about 10%) to the must to contribute stem tannins.
As a rule of thumb, winemakers consider skin tannins to be good and seed or stem tannins to be bad.
As the alcohol level rises, the tannin extraction profile changes. The compounds that dissolve in alcohol more than in water are also sometimes characterized as harsh tasting. They are also considered to be more reactive with anthocyanins, leading to greater loss of bright red colors. For those reasons, some winemakers prefer to press prior to the end of alcoholic fermentation, preserving a milder tannin profile and more youthful red color.
Tannin Polymerization
Throughout fermentation, the tannins that are extracted undergo other chemical reactions beyond the linking with anthocyanins. In the presence of oxygen, they form longer polymer chains much like the process that occurs in ripening grapes. In the absence of oxygen, they may form insoluble combinations that drop out in the lees. The tannin-anthocyanin complexes that are formed are red-brown in color and take the place of the brighter anthocyanins from which they form.
During aging, further polymerization of tannins goes on. This can be seen in the gradual conversion of red wine color to brick or tile hues as the remaining anthocyanins and tannins combine. Tannins may also continue to form complexes so large that they can no longer be dissolved in wine and fall out as sediment. Aging in oak further contributes to the wine tannin profile. Oak tannin structure can be viewed as being made up of gallic acid units and ellagic acid units — more phenol-derived compounds. As they develop in the oak tree, the tannins are built up through combining glucose molecules with these acids. The resulting tannins are fairly soluble in water and in wine. They may also be broken down (or hydrolyzed), freeing the original acid and glucose. That reaction path is the source of the term “hydrolyzable tannins” in reference to these compounds, as contrasted with the grape tannins that are considered the “non-hydrolyzable tannins.” The oak tannins provide further tannic structure to the wine, contributing to mouthfeel and possibly stabilizing color.
There are other phenolic compounds that are not tannins but can be extracted from oak. Vanillin, for instance, is the phenolic compound that represents the characteristic aroma of vanilla. Oak tannins may be introduced to the wine by barrel aging; the use of oak chips, beans, sticks, or staves; or the direct introduction of oak tannin powder.
Removing Tannins
In contrast to adding tannins to wine, the winemaker sometimes needs to remove them. A wine may be too harsh and astringent for early drinking and the winemaker may choose not to rely on barrel aging or long bottle storage to let the tannins combine, mellow, and fall out. To quickly remove a portion of the tannins, we can employ the same reactions that are used in tanning hides: tannins readily combine with animal proteins and render them insoluble. Introducing egg whites, gelatin or milk to the wine will immediately bind some of the tannins. Made insoluble, they drop out into the lees, allowing the winemaker to rack off a mellower, softer-tasting wine. Since the tannins are gone, however, the wine will not have as long a potential aging life as it would have had with those tannins present. It all depends on winemaker objectives for that vintage.
As noted earlier, tannins are generally low in white wines. A partial exception to that rule of thumb is found in barrel fermented or barrel aged Chardonnay. Since oak tannins will be extracted during barrel contact, the winemaker may also wish to assure that a balanced tannin profile will result. By using either a cold-soak period to introduced grape skin tannins, or by adding enological tannins, it may be possible to produce a wine that is pleasantly oaky with a full mouthfeel.
In most reds, tannins are much more prominent. All of the tannins are astringent, with smaller molecules exhibiting the most astringent character. Wine bitterness varies, but can generally be viewed as at least partly contributed by the tannins in the wine. If the wine is fermented dry, then any bitterness that may exist will not be balanced by sugar. Ethanol, however, also produces a sweet sensation when not at such a high level that it causes burning “heat” in the mouth. As a beverage, wine is high in acid, another characteristic that is more pleasant when balanced by sweetness. Taken together, these features tell us that for a given content of acid plus tannins, a wine must have sufficient alcohol to provide balance. If acid, tannins, or both are too high, the wine is considered harsh and unpleasant. If acid and tannins are too low, the wine is overly soft and “flabby.” Since there are no home tests for tannin content in wine, the winemaker must rely on taste and experience to make decisions about crushing, cold soaking, macerating, fermenting, pressing, and aging wine to achieve the ideal tannin profile for that vintage. If all the best efforts in processing still leave something to be desired, the winemaker can add or remove tannins as needed.
With some knowledge of tannin chemistry, these decisions may become more clear. The winemaker knows what the various tannins are, where they come from, and how they get into the wine. The changes during production and aging are less mysterious and the outcome may be more predicable. Ultimately, the tannic effects on color, flavor, and mouthfeel of the finished wine are more directly under the winemaker’s control.






