Testing for SO2: Keeping your wine safe
First, I need to clear up some persistent confusion about the meaning of the term “sulfite.” Among the elements, sulfur is unusual in that it readily adopts several different oxidation states. Names assigned by traditional chemistry naming rules give sulfur compounds different similar names based on that oxidation state. Sulfate ion, SO42-, is a combination of oxygen in its usual -2 oxidation state and sulfur in +6. This sulfate ion is common in the environment, does not provide any protection to wine, and when formed by dissolving sulfur trioxide, SO3, in water makes sulfuric acid, H2SO4 (which ionizes almost completely to 2H+ and the SO42-). Sulfite ion, SO32-, today’s topic, is similar but acts in very different ways. Here, the sulfur is in the +4 oxidation state and the corresponding acid would be sulfurous acid, H2SO3, produced by dissolving sulfur dioxide, SO2, in water.
Depending on the pH of the wine, any sulfite present is distributed into three equilibrium forms.
The –ate ending on the ion and the –ic ending on the acid represent higher oxidation state, while the –ite ending on the ion and the –ous ending on the acid are lower oxidation state. These names are why you variously hear winemakers refer to additions of sulfur, sulfite, or SO2, to their wine. Regardless of which way you say it or what chemical form you add it in, the result is the presence of sulfite ion and related compounds in your wine.
Depending on the pH of the wine, any sulfite present is distributed into three equilibrium forms. At lower pH’s, the un-ionized molecular sulfur dioxide, SO2, is present. At higher pH’s, there is a combination of hydrogen ion, H+, and the bisulfite ion, HSO3–. Moving to still higher pH yields a higher proportion of sulfite ion, SO32-. Of all these, molecular sulfur dioxide is the most potent protection against oxidation and spoilage. It is present in very small amounts at wine pH. When added together with other bisulfite and sulfite ions and not combined with any other compound, we call the combination “free” SO2. That is the chemical species we usually measure and then find molecular SO2 in a chart based on the pH and the free sulfite analytical result.
The common cellar compound potassium metabisulfite is named that because it does not contain sulfite exactly but it will when dissolved in water. That is, the “meta” means it is about to become a sulfite compound when you dissolve it in water (or wine). In simplest terms, that can be shown as:
K2S2O5 + H2O → 2K+ + 2 H2O3–
From there, the pH determines the distribution of the sulfite forms. The way this cascades down to the molecular sulfur dioxide protecting your wine is like this:
Suppose you dissolve 100 mg/L (ppm) of potassium metabisulfite in your wine. In that amount, you have added 57 ppm of sulfur dioxide equivalent. Of that, if you ran a “total sulfite” test, you would find somewhat less than 57 ppm due to various losses or binding with other compounds. Depending on pH and other components in the wine, somewhat less would appear in a “free sulfite” analysis. A tiny fraction of the free would be in your wine as “molecular” sulfur dioxide, which provides the maximum protection.
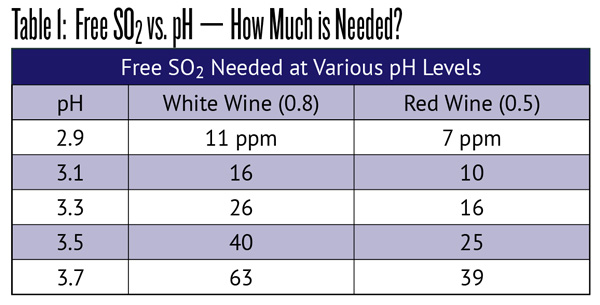
Why do we analyze for sulfite? Sulfites have two major beneficial effects on wine. Through interactions with naturally occurring phenolic compounds, sulfite reacts with oxygen, reducing the risk of oxidizing your wine (this reaction converts the involved sulfite into sulfate, removing it from both “total” and “free” SO2 test results). Sulfite also inhibits or kills spoilage organisms. This is so effective that native wine yeasts have evolved to be sulfite resistant and to produce sulfite in their metabolism — they inhibit competitive organisms in the rich sugar environment of juice. In both actions, molecular SO2 is the agent and free SO2 is the test. Winemakers generally agree that about 0.8 ppm of molecular is needed to protect white wine and about 0.5 ppm for reds. Reds need a bit less because there are other oxidation-resistant compounds present in red wine. Table 1 is a table of representative “free” levels needed for these target molecular levels at different pH values. You can also visit winemakermag.com/sulfitecalculator to obtain values for proper sulfite additions.

Although I usually make a sulfite addition to my juice or must on harvest day, there is no need for testing then. That initial dose to inhibit spoilage microbes will quickly dissipate and will be long gone by the time primary fermentation is complete. Then, my testing program begins. For wines that will go through malolactic fermentation (MLF), there is no addition of sulfite before the MLF bacteria inoculation to avoid inhibiting their growth. After MLF is complete, or after primary fermentation of wines without MLF, I make a first sulfite addition of about 50 ppm total SO2. After that, I make an addition every month during bulk aging. Using as a very rough guide that sulfite is used up at a rate of about ½ ppm per day, I make a 15 ppm addition monthly. I test the wines every other month and make another addition if the free SO2 does not conform to the target levels in Table 1 below. You will also want to make one final test and adjustment just prior to bottling.
The primary winery and commercial lab method of analysis has long been a test called aeration-oxidation. A quick but somewhat less accurate method called Ripper has commonly been used in winery cellars for routine monitoring. While both methods have had adaptations for home winery use, it was not until Dr. Richard Sportsman of Vinmetrica introduced his much-improved take on Ripper chemistry that a fast, easy, reliable home test became available. I do not usually cite single brand names in my column, trying to offer alternative products from different producers whenever possible. In this case, however, less than ten years ago Vinmetrica brought out this unique product that has no direct competitor. Revolutionary as it is for home winemakers, it still exploits the fundamental chemistry of the Ripper method.
In the August-September 2020 issue I described that titratable acidity (TA) is method-defined. The acid that titrates when you apply the method is, by definition, the titratable acidity. Sulfite, on the other hand, is a specific chemical species. A scientist can exploit any of its unique properties to render it amenable to analysis. Aeration-oxidation analysis depends on the volatility of sulfur dioxide gas and the development of an acid when reaction products are dissolved in solution. Ripper chemistry utilizes the oxidation of sulfite to sulfate by using iodine as a reactant.
Before Vinmetrica, Ripper was either a macro titration or a kit of vials. According to Zoecklein et al. in Wine Analysis and Production, free sulfur dioxide is measured by placing 25 mL of wine in a flask and adding a starch solution as an iodine indicator. The sample is acidified and then rapidly titrated with standardized iodine solution until the blue color change of starch indicates that all free sulfite has reacted. The answer is then calculated according to a formula that includes the concentration of the iodine. The kit version of Ripper is a product called Titrets. The manufacturer CHEMetrics states about Titrets, “This kit is not recommended for use with red wines or white wines containing ascorbic acid or tannin. These wines often give false high test results.” The same problem makes the Ripper titration method somewhat difficult to repeat with good precision. More specifically Titrets are sealed glass vials that contain colorless iodine-iodate reagent as a stand-in for standardized iodine. The vial also contains starch indicator and is packed under vacuum. Attaching a flexible plastic hose, the user breaks the vial neck and squeezes a glass bead in the tube, allowing the vacuum to suck up the wine sample. Since the amount of iodine is fixed, this amounts to a form of back titration and you keep adding wine until the endpoint is reached. In this case, the Titret reagent turns blue on the very first bit of wine entering the vial and then wine is added until sufficient sulfite has used up all the included iodine and the blue color disappears. The more wine it takes, the lower the sulfite concentration. The vial is marked along the side with free sulfur dioxide concentration in ppm.
Using similar chemistry, the Vinmetrica meter does not depend on visual detection of a starch endpoint. Instead, it utilizes a platinum electrode and is an amperometric titration. When a pair of electrodes is placed in a solution and a voltage is applied, the current that results depends on the composition of the solution. While this can be difficult to determine in an absolute sense, it is much easier to detect relative changes in the current in a changing solution. In this case, a small battery-powered meter applies a voltage between two platinum wires at the tip of the electrode. You add two Vinmetrica proprietary reagents to a wine sample, immerse the electrode, and begin titrating with an iodine-type proprietary titrant. When all of the free sulfite has reacted, the endpoint is reached and there is a sudden shift in the current. The meter indicates the sudden shift with a visual readout and an audible alarm. The combination of the proprietary reagents and amperometric method produce reliable, quick, easy results.
Before Vinmetrica, the best bet at home was aeration/oxidation or A/O. The wine sample is acidified to push the sulfite present toward the form of SO2 molecular that can be swept out of solution by bubbling air through it. The air stream is transported through a trapping flask. In that flask, hydrogen peroxide oxidizes the sulfite to sulfate, producing sulfuric acid in place. After 10 minutes of transfer, the air is stopped and the trapping flask is titrated to a specific pH color endpoint with sodium hydroxide titrant. Commercial all-glass setups for A/O are quite elaborate and can cost several hundred dollars. In recent years, home winemaking suppliers have introduced systems using ordinary flasks and silicone tubing, bringing the price down to about $100. Either kind of system can produce accurate, reproducible analysis for free SO2. Before I bought a Vinmetrica meter, I had purchased two different A/O setups. Both worked, but are a bit fussy to assemble and operate. They also involve the use of phosphoric acid and hydrogen peroxide. I now use my Vinmetrica meter exclusively to monitor my sulfite program. Whatever method you use, the important thing is to keep testing! Staying on a sulfite schedule is hugely important for the future safety of your wine.






