
Oxidation and oxidatively-driven degradation have been a constant plague on winemakers since time immemorial. Even today, with advancements in science and production, it continues to haunt producers of all sizes and styles.
Oxygen exposure is the driving force behind volatile acid production, mycoderma formation (film yeasts), acetaldehyde production, depletion of free sulfur dioxide (SO2), browning, pinking, loss of aroma or “freshness,” bottle shock, barrel spoilage (barrel shock), and reduction in shelf life.
Improper oxygen management, in my opinion, is the number one cause of wine quality degradation worldwide. There is a current feeling among consumers and some producers that SO2 usage should be reduced or eliminated from the production process. Aside from the fact that it is a natural byproduct of fermentative yeast metabolism, it is the most common anti-microbial and anti-oxidant available to winemakers. Its dualistic activity is very useful in the winemaking process. However, if a winemaker chooses to reduce or eliminate SO2, they must be hyper aware of their dissolved oxygen (DO) levels.
Most winemakers have some way to measure sugar, pH, titratable acidity (TA), and perhaps alcohol. These analytes are often considered to be the most important to measure throughout the process. Weighing the degradation risks, the prevalence of market wines with oxidative-related flaws, and my own experience over the years, I firmly believe that winemakers should also consider DO measurement a fundamental.
Over the years, winemakers have addressed oxygen-related problems in several ways; with various levels of success and unintended consequences. Some common techniques include inert gas usage, special handling techniques (gravity flow, certain pumps, pressure transfer, etc.), minimizing movements, and utilizing additives (SO2, tannins, and fining agents).
The small-scale winemaker faces more challenges than larger producers, simply due to batch size. As container size decreases, the surface area to volume ratio increases dramatically. Large commercial producers are by no means immune; they eventually bottle at 750 mL or smaller volumes, just like the rest of us. Winemakers have been aware of bottle shock for many years. Some have blamed filtration, pumping, dirty bottling lines, some even speak to the wine “finding itself” . . . but the underlying culprit is increased surface area and increased DO. The risks at bottling could not be higher; it’s the culmination of years of hard work. There is nothing more frustrating than having a problem in the bottle.
The importance of DO management is not a new topic, but it still seems to plague winemakers — professionals and hobbyists alike. The natural endpoint for grape juice is vinegar — we are trying to halt that progression and capture the most flavorful point in its evolution. Even in the technological age we live in, with all the resources on the internet, books and magazines that are available, interconnectivity of people; there is still confusion about DO. Ironically, I’ve seen commercial producers that have volatile acidity (VA) problems and no way to measure DO, who have invested thousands of dollars on micro-oxidation equipment.
Winemaking is, as we all know, an amalgamation of science and “art.” Some may say magic. Regardless, most would agree making wine is complicated; full of risk and reward situations and a perpetual balancing act. To address a technical topic such as DO in a concise and digestible way can often be a challenge. In this discussion we will investigate the stages of production, the tools and techniques for mitigation, and some basic chemistry and microbiology.
Stages of Production
Oxygen is not always a bad actor. It is vital in yeast metabolism, drives polymeric reactions (softening of tannins), can help stabilize color, and is critical in the production of certain styles of wine such as Sherry. Generally, yeast love oxygen; oxygenated yeast starters have higher viable cell counts and healthier fermentations.
There has long been a debate among white wine producers on whether brown or green juice is best. The green juice advocates often refer to this as “reductive” winemaking. The term reductive, unfortunately, is often misused. Reductive should be defined as an electro-chemical state of the wine, but is often used to describe aromas; commonly the perception of hydrogen sulfide, mercaptans, thiols, and disulfides. These aromas are a symptom of a complex system, and while a wine’s reductive state can drive their perception, it is not the cause.
Equipment manufacturers have made presses that completely inert the pressing atmosphere. Combining this with uber careful handling and exclusion of oxygen throughout the process can help drive certain wine styles, but also create very big problems. When a white or rosé wine is never exposed to oxygen there are certain compounds that never brown and drop out of solution. The risk here is during packaging, when container size radically decreases and surface area increases, browning or pinking in the bottle can occur.
Those winemakers that employ oxidation during the juice phase run a much lower risk of browning or pinking later. However, oxidation at this point is a double-edged sword, as it can drive the activity of certain spoilage organisms such as Klockera sp, Pichia sp, Hanseniaspora sp, and Candida sp. Most winemakers have experienced some ethyl acetate production; commonly described as nail polish remover. Warmer temperatures, absence of SO2, unsanitary conditions, compromised fruit quality, and delayed production can all increase the risk for this flaw to develop. Ethyl acetate is often associated with volatile acidity (VA), which is primarily comprised of acetic acid, but also butyric, propionic, and formic acids.
Generally, good manufacturing practices can prevent VA production. There are a few easy things that winemakers should keep in mind:
1. Temperature control — The microbes that cause VA thrive in warm temperatures. While cooler temperatures can help mitigate their microbial activity, DO solubility increases. This is the case with all dissolved gasses.
2. SO2 usage — Adding SO2 at the juice stage can help control spoilage microbes, while still allowing Saccharomyces to grow.
3. Timely processing — The sooner a fermentation starts the less chance for spoilage.
4. Sanitation — Judicious sanitation can reduce VA production by lowering the presence of viable populations of spoilage microorganisms.
5. Fruit Sorting — If there is the presence of damaged fruit, rot, and insects, physically separating these contamination sources is a viable route to prevent spoilage.
6. Implantation of commercial yeast — This can greatly reduce the proliferation of spoilage microbes. Some yeast strains can secrete inhibitory compounds that slow the growth of other microbes.
7. Acidulation — Generally speaking; lower pH inhibits the growth of spoilage microorganisms, increases the effectiveness of SO2, decreases the rate of browning reactions, and lowers the risk of metal content. Generally, ideal pH is between 3.1–3.55.
8. Eliminating ferrous metal — Metal content, specifically copper and iron, can greatly increase the deleterious effects of oxygen exposure.
Once fermentation is finished, DO control becomes even more critical. Every cellar action comes with some risk of increasing DO. This is a function of handling techniques, temperature, agitation, headspace, pH, and so on. Given the prevalence of oxidative degradation, it is my opinion that not only is the exclusion of oxygen important, but also the physical removal of it from the liquid
is paramount.
Tools and Techniques for Dissolved Oxygen Mitigation
Inert gas usage
There are three main gasses that can be used in the winemaking process. Inert gasses can be used to inert headspace, flush lines, push wine out of hoses, sparging, etc. Inert gasses are not created equal and have different functions and preferred applications.
1. Nitrogen —Less dense than air, functionally insoluble. Best used as a sparging gas, by using a sparging stone and bubbling the stone in the wine to “rip” out DO (and CO2). Since it is lighter than air, it is not best suited for headspace management.
Due to the insolubility of nitrogen, it is extremely effective in removing DO and dissolved carbon dioxide (dCO2). Efficacy is dependent on nitrogen purity, bubble size (smaller = more reactive), temperature, and gas flow rate. Sparging is a relatively simple operation, simply drop a sparging stone into the bottom of the wine and turn the flow of gas on. Concerns would be violent foaming (this happens when there is a lot of dCO2 in solution), and sanitation of the sparging equipment. Commonly, 3–5 volume changes of high purity nitrogen is required to lower DO from saturation (at 68 °F/
20 °C and 1 atm of pressure) to <0.5 ppm.
2. Carbon dioxide — Heavier than air, soluble at various temperatures and pressures. Suitable for inerting headspace, but not ideal for sparging. Can cause unwanted effervescence, along with pressure and vacuum issues in sealed containers. Available in dry ice form as well as a gas.
3. Argon — Heavier than air, functionally insoluble. Best choice for headspace management but it is more expensive than CO2.
Before we move on from inert gasses, a warning: With any inert gas there are inherent dangers of asphyxiation in confined spaces. Use caution. Also, ripping wine down with nitrogen can render the liquid a “sink” for soluble gasses such as CO2. If nitrogen is used to eliminate dissolved gasses, and then CO2 is used in the headspace, it can dissolve into the wine over time and create a vacuum and collapse a sealed container.

SO2 usage
1. SO2 is the most common anti-microbial and anti-oxidant preservative used in wine production.
2. Efficacy is dependent on pH, there are many resources online to determine the correct levels of free SO2 needed for microbial control. Debate continues regarding what proper molecular level is needed, but historically it is between 0.5 and 0.8 ppm molecular.
3. Since it is also an anti-oxidant, it will bind with DO (technically, it will bind with the peroxy radicals created by oxygen). Commonly, it is thought that 1 ppm free SO2 binds with 4–5 ppm DO. This partially explains why some SO2 additions vary in efficacy. The depletion of SO2 is intrinsically connected to DO levels. If DO is eliminated prior to an addition (via sparging), then the SO2 can persist primarily as an anti-microbial. The importance of this is amplified when the pH is higher, where the risk of bacterial growth is much greater.
4. SO2 exposure can be hazardous. Care should be taken, and personal protective equipment (PPE) worn when handling SO2.
5. Granular SO2, commonly referred to as KMBS (potassium metabisulfite) or Campden tablets, lose efficacy when stored for long periods of time. This loss in efficacy is exacerbated by exposure to air and humidity. A vacuum sealer is a powerful tool to preserve partial quantities of any product.
6. There is a belief some have that they are allergic to SO2. While there may be people “sensitive” to exposure, there is growing skepticism that people are indeed allergic. Commonly, these people indicate they have an intolerance to red wine but can drink white wine without major symptoms. This presents a dichotomy. Firstly, white wines typically have higher levels of SO2, because they have less tannins and lower alcohol, and often have not gone through malolactic fermentation (MLF). Red wines, on the other hand, commonly go through MLF and have lower levels of SO2. Often, this is done by native species of lactic acid bacteria. Native species often produce biogenic amines (histamine, tyramine, putrescene, cadaverine), while most commercially-available strains do not have the ability to produce biogenic amines. Note the histamine; this is thought to be reason for people’s “allergic” reaction to red wines, not SO2. Oddly enough, people have turned to “natural” wines to avoid SO2, and since natural wines are commonly made with native microorganisms, the risk for biogenic amines is much higher, as are allergic type symptoms.
7. SO2 content, depending on a wine’s composition, can be perceived sensorially. Generally, it is thought that levels above 60 ppm free SO2 can render a wine unpleasant and may cause a sensitivity reaction in
some people.
Measuring/knowing your dissolved oxygen
1. Measurement — Dissolved oxygen can be measured with a DO meter. There are many different models available on the market at various price points. Cheaper models typically do not have low % precision to quantify low levels (<1 ppm DO).
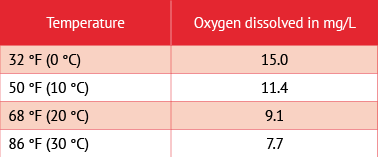
2. Theoretical — By measuring atmospheric pressure and the wine’s temperature, one can calculate the maximum solubility of oxygen (see chart, below).
Tannins
1. Generally, the greater the tannin content, the higher the resistance to chemical oxidative degradation (as such, red wines have better resistance compared to white and rosé wines).
2. Exogenous tannins can be added at various stages of production to increase the anti-oxidant capacity of a given wine. Tannins can be derived from many different sources such as gallnuts, various woods, grape skins, grape seeds, etc.
3. It is possible to add too much tannin. Over-addition can render a wine bitter and astringent. Care should be taken to prevent this, and bench trials are always recommended to determine the correct addition.
Specific inactivated yeast (SIY) products/lees
1. SIY’s can simulate the antioxidant protection that lees aging has shown in the past, without the microbial and sensory risks associated with native lees aging.
2. Products such as Lallemand’s Pure Lees Longevity has been shown to absorb 1.7 mg/L of DO at an addition rate of 40 g/hL.
Copper sulfate usage
1. Copper sulfate (CuSO4) can be used to bind and eliminate volatile sulfur compounds (VSCs) such as hydrogen sulfide, thiols, and mercaptans.
2. Since copper is a very powerful oxidative catalyst, care should be taken in the usage of copper. CuSO4 also has a legal limit as defined by the TTB. Bench trials should be conducted to determine correct levels needed to correct the problem and ensure legal limits are not exceeded.
Handling
1. Less intervention — For DO mitigation, it is ideal to move/
rack/handle the wine as few times as possible to prevent DO uptake.
2. Barrel storage/maintenance — While it is common to inert tanks and other containers prior to filling, gassing a barrel before filling is less common. A lot of spoilage occurs during barrel aging. There are a few reasons for this. First, if the barrel is not inerted prior to filling, the wine encounters air and the free SO2 is depleted as a result, lowering its antimicrobial efficacy. Secondly, the barrel is by nature a septic environment. Third, surface area/oxidation increases due to breaking a larger batch down into barrel quantities. Lastly, winemakers top up on a very regular basis to “prevent oxidation.” If you have ever pulled off a silicone bung from a barrel, there is a noticeable “whooshing” sound that occurs. This is because the wine has evaporated somewhat but also soaked into the wood. This is known as the angels’ share. When this happens, a vacuum is created. By definition, there is no oxygen in a vacuum. Therefore, winemakers are often doing the opposite of their intention. Ideally, if a winemaker sparges the wine destined for barrel and then makes an appropriate free SO2 addition, gasses the barrel before filling, and conducts a less frequent topping regime then the stage is set for maximum efficacy of the SO2, while oxidation and spoilage risks are reduced.
3. Headspace minimization — This is a well-known technique, and usually is accomplished by either reducing the volume by putting the wine in a topped up container, or removing the air in the headspace using inert gas. Many winemakers use variable capacity tanks to mitigate the volume of headspace. There are two concerns with these tanks that should be taken into account when using them. First, the sealing tube is pressurized with air to make a seal and it is common for it to deflate. Second, while the seal is an adequate liquid barrier, it is not a great gas barrier, and there can still be air migration around the seal.
4. Bottling — Most hobbyists use a hand corker, while professionals typically use a vacuum corker. This is an important distinction. Care must be taken using a hand corker to properly inert the bottle using nitrogen or argon prior to filling. Since the cork is not inserted under vacuum, the composition of gas in the headspace in the neck of the bottle will be pressurized when the cork is inserted using a hand corker. This can greatly exacerbate bottle shock.
Fining agents
Fining agents can be used to mitigate or even rectify oxidative degradation. This is accomplished by four basic mechanisms:
1. Using a fining aid such as PVPP to remove the polyphenolics involved in browning and pinking reactions.
2. Using a fining aid such as certain chitosan formulations to remove the metal catalysts involved in oxidation reactions.
3. Using a fining aid such as isinglass or gelatin formulations to agglomerate and precipitate the microbial populations responsible for spoilage, followed by racking or filtration.
4. Using a fining aid such as carbon or PVPP formulations to remove the oxidized color components from a wine that has suffered chemical oxidation.
Key Takeaways
1. Oxygen is inversely soluble with temperature.
2. The risk of dissolved oxygen increases as the container size decreases.
3. Using inert gasses, and especially nitrogen sparging, are effective tools to mitigate DO.
4. Controlling DO will minimize spoilage and increase the effectiveness of SO2.
5. Controlling DO will greatly improve a wine’s shelf life.






