
An understanding of what is happening in wine on a chemical basis can be very useful in influencing choices regarding processing options and timing of activities for different wine styles. Unfortunately winemaking chemical nomenclature, chemical analyses, and interpretation of results can be daunting for those without a background in chemistry. Perhaps one of the most complicated subjects in wine chemistry is phenolics, the chemistry of phenolic aroma molecules, anthocyanins, and tannin. I received an undergraduate degree in chemical engineering, yet even for me it has taken many years and much study to wrap my head around how phenolic chemistry impacts processing options for different wine styles. I’m still learning, and there are research questions yet to be answered, but in a series of articles I will try to discuss the topic in ways that give home winemakers useful information in determining the best processing options for the wine styles they produce.
Let’s begin with a basic rundown of the chemistry of phenolic and polyphenolic molecules. Starting with the most basic structure, a phenol is defined as a six-carbon ring with a hydroxyl (-OH) group attached (See Figure 1). Phenolic molecules are plentiful in plants and have a wide range of uses including: Components in the structure of the plant, antioxidant and UV resistance, pest repellent properties, and signaling. The carbon ring structure of this molecule is important because it allows the carbon molecules in the ring to “share” electrical charge from the oxygen atom attached to it, which makes it easier for the entire structure to engage in chemical reactions with other molecules.
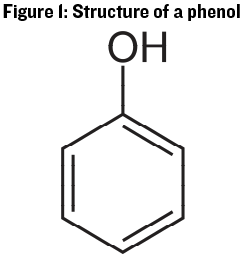
While there are some small phenolic compounds in wine similar in size to the basic phenol group, most of the interest in quality winemaking is on compounds with multiple phenolic ring structures such as anthocyanins and tannins. Compounds with multiple phenolic rings are referred to as polyphenols. Anthocyanins are the molecules responsible for wine color, and “tannin” is a catch-all term for a large group of compounds that impact red wine bitterness and astringency. In the following paragraphs we’ll move from a discussion of the smaller aromatic phenols up to the larger polyphenols, focusing on the factors important to winemaking in each group.
Aromatic Phenols
Small phenolic molecules have low mass and are hydrophobic due to their carbon ring structure, thus they have a low enough vapor pressure that they are volatile and at relatively low concentration may be recognized in wine. Aromatic phenols in wine come from a variety of sources. They are sometimes referred to as spoilage aromas because Brettanomyces metabolism creates a range of volatile phenolic compounds, the two most widely studied being 4-ethylphenol (4-EP) and 4-ethylguaiacol (4-EG). These smaller phenolic molecules are produced from the breakdown and rearrangement of larger non-flavonoid phenols (discussed next). 4-EP smells of Band-Aid/antiseptic and 4-EG has a barbecue/smoky aroma, but combined with other volatile phenols the aroma of Brettanomyces can range from sweaty saddle leather to horse barn. Phenolic molecules have antiseptic properties and were some of the first compounds used to inhibit wound contamination in hospitals, which is why we associate the smell of some phenolic compounds with antiseptic/hospital aromas.
Oak cooperage is another common source of volatile phenols in wine, yet these aromatic phenols are generally more well-liked than the phenols of Brett metabolism. Oak volatile phenolic molecules may be generated by the tree itself or by the breakdown of larger polyphenolic molecules in wood lignin during seasoning and toasting. Examples of aromatic phenols in toasted oak are vanillin and eugenol, which smell like vanilla and cloves, respectively.
Another recently recognized source of aromatic phenolic molecules in wine is from smoke taint. Volatile phenols from burning vegetation such as guaiacol and 4-methylguaiacol may impart a charred, ashtray aroma to wines made from smoke-tainted grapes.
Finally, chlorinated phenolic molecules are the precursors to the production of 2,4,6 trichloroanisole (TCA), the compound most associated with cork taint. While TCA is not considered an aromatic phenol due to its modified structure, it is produced by a range of microbes when they are exposed to chlorinated phenolic molecules often found in wood preservatives or bleached wood products.
Non-Flavonoid Phenols
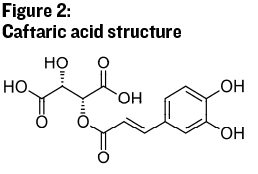
As mentioned earlier, the molecules that provide color and astringency are arguably the most important groups of phenolic molecules in wine. Anthocyanins and tannins fall into a general group of polyphenolic molecules called flavonoids, which will be discussed next. The non-flavonoid phenols then, are phenolic molecules that do not have the structure of the flavonoids. Some of the most important molecules in this category are a group of organic acids known as hydroxycinnamates (see Figure 2). These acids are found in both red and white juices. They are easily oxidized and thus can be an indication of oxygen exposure in a young must or wine. As mentioned, hydroxycinnamates may also be broken into smaller molecules through the activity of spoilage microbes such as Brettanomyces, becoming aromatic phenols. Added enzymes may also convert hydroxycinnamates into aromatic phenols, so proper enzyme selection is important if commercial enzymes are added during crushing and pressing.
Other non-flavonoid phenols include stilbenoids, a family of molecules that are considered antioxidants and have been studied for their potential health benefits. Resveratrol is the most famous molecule in the stilbenoid group. Stilbene levels increase with exposure to spoilage microbes and sunlight. Because of their correlation with sun exposure, and because sun exposure has a positive impact on many other wine quality parameters, resveratrol concentration is considered to be positively correlated with wine quality.
Flavonoids
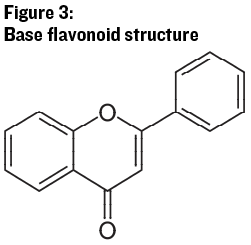
Flavonoids are distinguished by their three ring structure (see Figure 3). This is where the discussion of anthocyanin and tannin molecules begins to get interesting, and complicated. There are a host of different molecules, with different properties, which originate from this base structure. The rings have a variety of different hydroxide (-OH) groups bonded in different positions, which lead to different physical properties (such as the appearance of color) and different reaction kinetics with other molecules in wine.
The base group may also be attached to one or more molecules of glucose, called glycosylation, which again changes the name and reaction characteristics of the molecule. In addition, many of these molecules are often bonded together in oligomers (a few repeating units of structure) or polymers (many repeating units of the same or similar structures), which are also reactive and may add or subtract units or change structure. Finally, these molecules can react with other chemicals in the wine matrix such as sulfur dioxide, hydrogen sulfide and other sulfur-containing molecules (thiols), and acetaldehyde to either append these molecules onto the base flavonoid or to change the structure of the flavonoid and create other compounds. The variability of these molecules and their polymers, and the changes that occur in wines over time, make the study of wine processing impacts on flavonoid chemistry and the sensory impact of these changes quite challenging. Next we’ll dive into the flavonoid subgroups important for wine production and discuss the important chemistry of these diverse molecules.
Flavonols – Quercetin
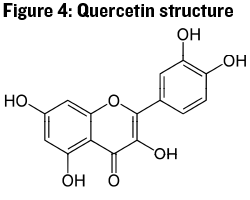
Flavonols are a class of flavonoids that have the basic shape shown in Figure 3, but each particular molecule has added hydroxyl (-OH) units, and possibly glucose molecules, bonded onto the base ring structure. The example shown in Figure 4 is one of the most common flavonols in wine, Quercetin. Quercetin acts as a sunscreen for grape berries by absorbing UV light. Thus this molecule, like the stilbenoids, shows increasing concentration in berries with increasing exposure to UV light. Flavonols are an important cofactor in the co-pigmentation of anthocyanins (discussed next), and may also contribute to color stability. There may be more to the story with flavonols and quality wine production, but research into this class of compounds and their impact on wine has been somewhat limited.
Anthocyanins
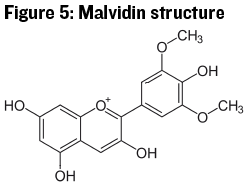
Anthocyanins, the color molecules in the flavonoid group, have a slightly modified structure from the flavone backbone above. Here the oxygen molecule at the bottom of the middle ring has been removed and the oxygen molecule at the top of the middle ring has a positive charge (see Figure 5). These structural changes make anthocyanins colored because of the alternating single and double-bonded carbon atoms in all three rings (in chemistry called conjugate double bonds or conjugated pi bonds). There usually needs to be at least eight of these alternating single and double bonds for a compound to absorb color in the visible spectrum, so any disruption to the anthocyanin structure that disrupts the conjugate double bonds will cause the molecule to lose the ability to absorb visible light, and thus appear colorless.
Anthocyanins at typical wine pH levels can exist in several different forms, some colored and some colorless. The colored version of the compound is in equilibrium with several colorless forms, such that only about 25% of the total anthocyanin is typically in the colored form at wine pH (Jackson, 2014). One of these colorless forms is caused by the addition of bisulfite ion to the middle ring, known as sulfite bleaching. This condition is temporary, and as bisulfite ions interact with other molecules in solution and become oxidized, the bisulfite will drop from the anthocyanin and the anthocyanin may revert to the colored form.
Early in the life of a wine anthocyanins may be found as single molecules or in stacked arrangements with other anthocyanins and phenolic molecules to form “co-pigmented color.” The ring structure of these compounds, and the tannin molecules discussed next, make them hydrophobic. This hydrophobicity favors these types of molecules clumping together into colloids, which is how the stacking of co-pigmentation might be imagined to occur. Co-pigmentation increases the depth of color beyond what would be expected from the individual anthocyanins alone, and also shifts the color hue to a slightly more blue color. As wine ages, anthocyanin molecules may: Be oxidized to colorless compounds, interact with acetaldehyde or pyruvic acid to form stable color, or polymerize with tannin molecules to become locked into what is termed polymeric pigment. The molecules resulting from anthocyanin interaction with acetaldehyde or pyruvic acid are called pyranoanthocyanins and have several subgroups into which they may be also be named (e.g. vitisins, pinotin). Pyranoanthocyanins are color-shifted to a more brick red or slightly orange color, which is part of the reason older wines may take on this hue. Pyranoanthocyanins are relatively stable and are not bleached by sulfites. Anthocyanins that become locked into polymeric pigments are also stable from a color perspective, thus polymeric pigment is thought to be an important component for achieving long-term color stability.
A couple of final notes on anthocyanins that we will explore in future articles: For most grape varieties almost all the color may be found in the skins of the grape, which has a big impact in processing decisions for extracting color into, or keeping color out of, juices and wines. And finally, the anthocyanins in hybrid grape cultivars are often different from the anthocyanins in Vitis vinifera grape cultivars. Hybrid wines may have up to 100% of their anthocyanins in a diglucoside form, which means there are two glucose molecules attached to the main flavonoid structure of the anthocyanin instead of the single glucose molecule in vinifera cultivars. This second glucose molecule appears to limit the ability of hybrid anthocyanins to form pyranoanthocyanins (one of the stable forms of color) and also greatly slows the reactions that incorporate anthocyanins into stable polymeric pigments (Burtch & Mansfield, 2016). Couple this with the generally low level of tannin in hybrid red wines and it becomes clear that hybrid winemakers have an uphill battle in making deeply colored and color-stable wines.
Polyphenolic monomers and polymers — Tannin
We’ll end our discussion of polyphenolics with the most important group for red wines, the monomers and polymers that make up what we refer to as tannin. The base structure of tannin in wine is usually described as the catechin unit, shown in Figure 6.
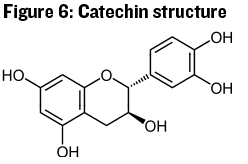
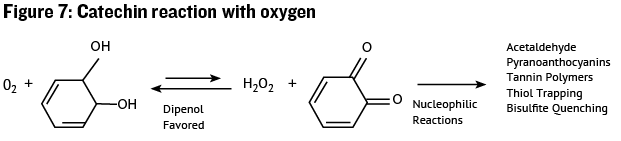
This molecule looks a bit similar to an anthocyanin, but the really important difference is the two hydroxyl (-OH) groups located on adjacent carbon atoms in the separated ring structure on the right in Figure 6. This structure is referred to as a vicinal diphenol, and it may react with oxygen to produce an o-quinone (see Figure 7).
(Adapted from: Waterhouse, et al. 2016)
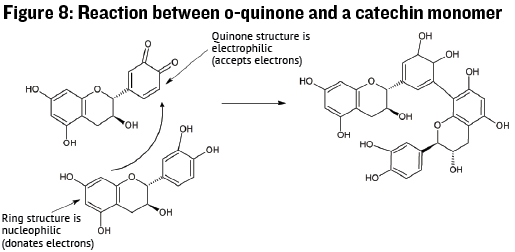
While the diphenol is not very reactive, the o-quinone is very reactive once formed and may undergo chemical reactions with many other molecules including: Anthocyanins, thiols, acetaldehyde, proteins, and other catechin units. When the o-quinone reacts with another molecule it forms a bond to the molecule that may be permanent, such as in tannin polymerization, but can also be temporary. If the bond is temporary the o-quinone will be regenerated to the catechin form and the other molecule will be oxidized to some other form. Thus the catechin unit can act as a powerful although somewhat slow antioxidant, removing oxygen from the wine matrix and reacting with other molecules to produce a wide range of reaction products. In Figure 8 we see an example a reaction between an o-quinone and a catechin monomer, yielding a two-unit “tannin” product. This is an example of how small catechin sub-units may combine to form larger tannin polymers.
(Adapted from: Waterhouse, et al. 2016)
To make things even more complicated, a relatively small fraction of the total polyphenol content in grapes and wine exists in the catechin monomer form. More often catechin is bonded to itself and other polyphenolic variants in groups from about 2 to 100 subunits in length (see Figure 9).
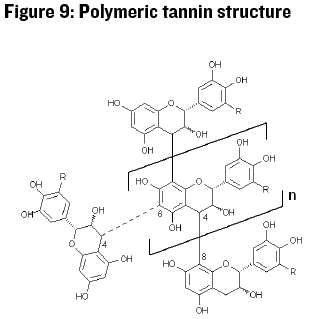
Catechin-based polymers have different sensory properties depending on the size of the tannin polymer. Large polymers interact with proteins in saliva, binding or precipitating the proteins and thus giving the feeling of roughness or dryness. Smaller polyphenols have lower astringent impact but can taste bitter. Tannin polymers are found primarily in the skins and seeds of grapes. The polymers in grape skin tend to be larger (3-83 subunits) than the polymers in grape seeds (2-16 subunits) (Johnson, 2016).
The sensory differences between seed tannins and skin tannins might suggest that skin tannin extraction should be encouraged and seed tannin extraction avoided. But seed tannin is important for properly structured reds, with tannin experts showing that as much as two-thirds of the total tannin in red wines is extracted from the seeds (Kennedy, 2008). That being said, grape seeds contain an extraordinary amount of tannin, so crushing seeds to extract more tannin is definitely not a good idea. The myriad winemaking process variables impacting the extraction of tannins from the skins and seeds into wine is something we will cover in a future article.
The general ability to extract catechin and tannin from skins and seeds changes as the berry ripens and the seed coat hardens. Less ripe fruit tends to be higher in catechin monomer, not the polymeric form (ETS Laboratories, 2020). There are also differences in the amount of catechin in different cultivars, including in hybrid grapes. Catechin level in wine is thus a complicated matrix of the grape cultivar, the ripeness of the fruit, and winemaking processes. In addition, tannin chemistry changes as the wine ages and oxidation reactions increase the size of tannin molecules and produces reaction products with the other chemical components in wine. Both catechin monomers and polymers may participate in the oxidation reactions and condensation reactions (reactions that form larger tannin polymers) that were discussed in earlier paragraphs. These molecules are most reactive when they are first extracted, during fermentation and the early part of wine aging. Thus the addition of oxygen in winemaking during these early stages may actually encourage color stability and tannin polymerization into forms that are thought to be “softer” and “rounder” than the aggressive tannins in young wines.
Catechins also have a role to play in white wine production. Catechin levels have been linked to the level of yellowing and browning during aging of white wines. Catechin appears to form linkages with non-flavonoid phenol molecules, and other catechin molecules, which eventually lead to large molecules with enough conjugate double bonds to absorb visible light. Catechin levels in white wines are much lower than reds because whites are pressed immediately. However, there can still be enough catechin, especially in hybrid grapes, to cause issues with premature yellowing and browning.
The complexity of tannin polymers and the products of their chemical reactions in wine make research in the area of tannin chemistry difficult. It has also been difficult to interpret how tannins of different size and type impact human perception. Winemakers and researchers describe tannins in ways that can be difficult or impossible to replicate with chemical standards, thus making training in tannin perception a bit of a metaphysical exercise (Oberholster, 2019). Another complicating factor is that tannins and their perception also exist within the wine matrix. Research has shown that other parameters in wine, like sweetness, acidity, and ethanol level can impact the perception of tannin. There is much still to learn regarding these complex polyphenolic molecules and how humans perceive them in a mixture as complicated as wine.
If you’ve found yourself lost in the complex polyphenolic web of chemical structures and nomenclature, don’t lose hope. I’ve included references in this article to several websites and papers that also explain the role of polyphenols in winemaking and contain many more figures and graphics, so you may find your footing with help from these resources. In future articles we will move beyond the fundamental chemistry described here and discuss how specific winemaking processes impact the extraction and reaction of polyphenols, particularly color and tannin. Then we can focus on the optimum processing methods for producing specific wine styles.
References
• Burtch, C., & Mansfield, A. K. (2016, August). Comparing Red Wine Color in V. vinifera and Hybrid Cultivars. Retrieved from Appellation Cornell – Research Focus 2016-3b: https://grapesandwine.cals.cornell.edu/sites/grapesandwine.cals.cornell.edu/files/shared/Research%20Focus%202016-3b.pdf
• ETS Laboratories. (2020). Phenolic Composition – Profiling phenolic compounds in red wine. Retrieved from ETS Laboratories: https://www.etslabs.com/library/34
• Jackson, R. S. (2014). Wine Science, Principles and Applications. Academic Press.
• Johnson, C. (2016). Tannin – Key Factors in Red Wine Taste and Mouthfeel. Retrieved from UC Davis Waterhouse Lab – What’s in Wine?: https://waterhouse.ucdavis.edu/whats-in-wine/tannin
• Kennedy, J. (2008). Grape and Wine Phenolics: Observations and Recent Findings. Ciencia e investigación agraria, 35(2), 107-120. Retrieved 01 10, 2020, from https://scielo.conicyt.cl/pdf/ciagr/v35n2/art01.pdf
• Oberholster, A. (2019, February 21). Wine Mouthfeel and the Mouthfeel Wheel. Retrieved from University of California Agriculture and Natural Resources: https://ucanr.edu/repository/fileaccess.cfm?article=177489&p=HKTJEW
• Waterhouse, A. L., Sacks, G. L., & Jeffries, D. W. (2016). Wine Oxidation. In A. L. Waterhouse, G. L. Sacks, & D. W. Jeffries, Understanding Wine Chemistry (pp. 283-285). Chichester, West Sussex: John Wiley & Sons, Inc.






