Prevent Volatile Acidity
 Volatile acidity (VA) is probably one of the most common wine faults encountered in home wine production. The reason VA occurs so commonly is partly due to the small production volumes encountered in home winemaking. These small volumes are more susceptible to oxidation, and oxygen is the main ingredient in the chemical and metabolic reactions that result in VA. Before we discuss how VA occurs and is detected we should briefly discuss what volatile acidity is and how the volatile acid in wine differs from the fixed acids, which are not volatile.
Volatile acidity (VA) is probably one of the most common wine faults encountered in home wine production. The reason VA occurs so commonly is partly due to the small production volumes encountered in home winemaking. These small volumes are more susceptible to oxidation, and oxygen is the main ingredient in the chemical and metabolic reactions that result in VA. Before we discuss how VA occurs and is detected we should briefly discuss what volatile acidity is and how the volatile acid in wine differs from the fixed acids, which are not volatile.
What is Volatile acidity?
Complex mixtures of chemicals in liquid solutions are bound in the matrix of the solution due to their relative attraction to the other chemical species that make up the solution. The attraction or repulsion of different species is impacted by the size and shape of the molecule, and also the partial positive, negative, or neutral charge of the molecule in relation to the other molecules in the matrix. Molecules that are large or highly attracted to other molecules in the liquid matrix tend to stay in solution. Molecules that are small and/or are repelled from most of the molecules in the matrix can attain enough energy to leave the liquid and become a gas. The tendency of a given molecule to evaporate, leaving a liquid matrix, is measured as vapor pressure. Molecules with a high vapor pressure will easily evaporate from the liquid and are often referred to as volatile. The concentration of these molecules in the air space above the liquid surface partly determines whether or not we can recognize them as an aroma when we sniff wine in the glass. Another factor involved in whether or not we can smell a particular chemical is the human recognition threshold for the given chemical.
Some chemicals are easily recognizable in solution concentrations in the parts per trillion range (e.g. molecules like trichloroanisole, which is responsible for cork taint aroma). Other chemicals are not recognized, on average, until reaching hundreds of parts per million in solution concentration (e.g. acetic acid, part of the matrix of volatile acidity compounds).
Matrix effects also play a role in whether or not we smell a particular chemical; some chemicals may be additive in their ability to be recognized by humans, other compounds may be subtractive and mask the smell of other chemicals. Still, whether or not we smell a particular aroma in wine is largely due to the vapor pressure of the chemical and our aroma threshold for that chemical.
Thankfully, grapes contain a number of acids that impart a tart taste to the juice and wine, and these acids also inhibit growth of some bacteria and yeast. Most grapes contain fairly large quantities of tartaric and malic acids, in the grams per liter range, and a small amount of citric acid, in the tenths to hundredths of grams per liter range. These acids have a large enough molecular weight that they are essentially nonvolatile in juice and wine; meaning they do not readily break free from the liquid surface of wine and thus do not have a high enough concentration in the headspace above the wine for us to smell.
Several other acids in wine are generated by the action of yeast or bacteria. Lactic acid, generated by lactic acid bacteria, is produced from the conversion of malic acid during malolactic fermentation. Succinic and acetic acids are also generated from the metabolism of either yeast or bacteria. All of these acids can impart a sour taste to wine, but acetic acid is the type we will focus on because it is small enough to readily evaporate from the wine matrix and therefore have an aroma.
Causes of Volatile acidity
Acetic acid is one of the smallest carboxylic acid compounds in nature, with only one carbon atom attached to the carboxylic group. It is recognized as the sharp, pungent aroma found in vinegar. Acetic acid is a metabolic by-product of both alcoholic fermentation by yeast and malic acid degradation by lactic acid bacteria. Thus some acetic acid will be generated in normal wine production, perhaps 0.1–0.5 g/L (100 to 500 mg/L). Many factors impact the level of acetic acid produced and include: Yeast and lactic acid bacterial strain, temperature, and starting Brix. Fortunately, acetic acid has a relatively high recognition threshold in wines, with wine texts giving 200 mg/L as the average detection concentration.1 Most winemakers I’ve spoken with are usually not overly worried about acetic acid levels in red wines until they rise above 700 mg/L. Wild yeast or bacterial strains, or high starting Brix (such as Botrytized or icewine production), however, can push the metabolic production of acetic acid above 1,000 mg/L.
Acetic acid may also be generated after alcoholic fermentation from the oxidation of ethanol by acetic acid bacteria. This conversion of alcohol to acetic acid is the traditional method of vinegar production. Unlike yeast and lactic acid bacteria, acetic acid bacteria require access to oxygen in order to convert ethanol to acetic acid. Thus both the active bacteria and access to oxygen are needed for production of acetic acid in wine (see Figure 1, below).
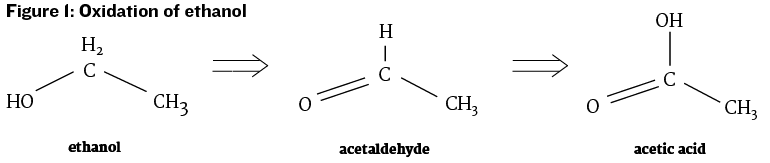
An intermediate chemical in the oxidation pathway from ethanol to acetic acid is acetaldehyde. Acetaldehyde is often described as smelling like Sherry. In Sherry production acetaldehyde is produced by film yeast allowed to grow in partially-filled barrels. Acetaldehyde is also described as having a nutty or overripe apple aroma. Acetaldehyde may be produced either through microbial metabolism or from metal-catalyzed chemical oxidation. As such, acetaldehyde aroma is a general marker of oxidation in wine. Acetaldehyde has a moderately low aroma threshold of 100 mg/L, but at low levels smells mildly fruity.2 Acetaldehyde readily binds with SO2 in wines where SO2 has been added, and this bound acetaldehyde is no longer volatile and therefore does not contribute to wine aroma.
Microbial metabolism is one way of increasing the volatile acidity in wine. Another is chemical oxidation, which we will briefly discuss next. A third method, enzyme-catalyzed reactions, can be important in browning reactions in juices but these enzymes are deactivated in alcoholic solutions and thus are not seen in finished wines. Chemical oxidation occurs in wine through the activity of oxygen creating hydrogen peroxide and peroxide radicals, and reaction of the radicals with any compounds in wine susceptible to oxidation. The conversion of oxygen to hydrogen peroxide and peroxide radicals is facilitated through electron exchange between metal ions like copper and iron and phenolic compounds. The metal ions and phenolic compounds act as catalysts because they are easily regenerated to their original state, thus they do not have to be present in large quantity in order for chemical oxidation to occur. In fact grapes, even white grapes, contain enough metal ions and phenolic compounds for chemical oxidation to occur.
There is one more critical chemical reaction involved in the discussion of volatile acidity. In an acidic environment like wine, it is possible for acetic acid to react with ethanol to form an ester. Esters are a group of chemical compounds that have a carboxylic acid group linked to another organic molecule by the oxygen atom from the OH bond of the acid group. Low molecular weight esters are often volatile. If acetic acid is present in wine a portion of it will esterify with ethanol to form ethyl acetate. Ethyl acetate smells like solvent or nail polish remover. It has a much lower odor threshold than acetic acid, reported as low as 12 mg/L.3
Because the aroma threshold of ethyl acetate is so much lower than acetic acid, this solvent or nail polish remover smell is often the first sign of microbial oxidation in wine. At low levels ethyl acetate, like acetaldehyde, has a slightly fruity aroma. But at higher levels the solvent aroma can overpower any other aromas in the wine.
Preventing Volatile Acidity
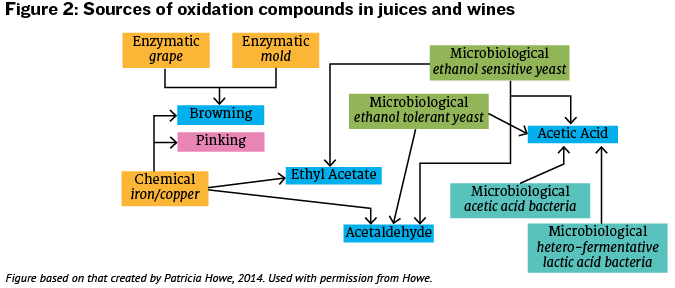
So, volatile acidity as a term represents the accumulation of several different compounds in wine that may be produced through microbial metabolism and chemical oxidation. The sources involved in the generation of these different aroma compounds are summarized in Figure 2. This can make it difficult to troubleshoot the exact cause of VA problems in wine, but there are a number of prevention steps that may be taken to limit the possibility of wine spoilage due to volatile acidity.
During the pre-fermentation period it’s possible to minimize the growth of spoilage yeast and bacteria by proper vineyard management, sorting out damaged fruit, adding potassium metabisulfite, and either storing the must at low temperatures (less than 50 °F/10 °C) or limiting the amount of time prior to alcoholic fermentation. In warm climates it is beneficial to add acid to keep the must pH at or below pH 3.5, as several species of lactic acid spoilage bacteria have difficulty functioning at lower pH. If a low pH isn’t possible due to stylistic or other factors, then winery sanitation and use of commercial yeast will be beneficial for reducing the possibility of high VA levels from wild yeast or lactic acid bacteria.
Vineyards on the East Coast must contend with the possibility of large amounts of acetic acid being produced in the vineyard. Sour rot is a combination fruit fly injury and acetic acid bacterial metabolism that is quite common in eastern vineyards. Fruit affected by sour rot may bring appreciable levels of acetic acid into the juice before fermentation begins. Bird and wasp damage may also cause acetic acid production in vineyards anywhere. Proper vineyard management and fruit sorting at harvest are good practices to minimize acetic acid in grape must prior to fermentation.
Some limited amount of acetic acid and ethyl acetate production prior to fermentation may be mitigated during the fermentation process. Ethyl acetate is quite volatile and some studies estimate that as much as 50% of it may be entrained with CO2 during fermentation and lost. Acetic acid is not as volatile, but more research suggests that some amount of acetic acid may be metabolized by yeast during fermentation. Thus a small but noticeable amount of VA prior to fermentation may be mitigated by the fermentation process. However, high levels of VA prior to fermentation will not be impacted enough to eliminate their presence post-fermentation.
For alcoholic fermentation the best practice to limit the possibility of volatile acid generation is inoculation with a commercial Saccharomyces strain as these strains have been screened for low production of VA. If you are interested in splitting the difference between un-inoculated and inoculated fermentation there are now several strains of non-Saccharomyces yeast on the market that may be added prior to Saccharomyces. These non-Saccharomyces species are thought to give added aromatic complexity to wines in which they are used, but again they have been screened to limit VA and other off-odor production.
Malolactic fermentation (MLF) is another process where use of a commercial strain helps insure that the conversion of malic to lactic acid occurs in a timely manner and with limited VA and off-odor generation. Timeliness of MLF completion is important because the new wine is unprotected during MLF due to malolactic bacteria being sensitive to sulfites, and thus use of potassium metabisulfite is precluded until MLF is complete. Slow or stuck malolactic fermentation allows a window of time where other lactic acid bacteria (Pediococcus is a common example) may grow and produce high levels of volatile acid compounds.
Post-fermentation aging in carboys, tanks, or barrels is another area where volatile acidity may become a problem. Lactic acid bacteria, some yeast species, and acetic acid bacteria may grow in or on the surface of wines during aging. Two precautions will minimize the ability of these microbes to grow. First, limit access to oxygen by eliminating ullage (air space in containers) and keeping lids and bungs tightly sealed. Second, maintain adequate levels of free SO2 in the wine to inhibit microbial growth.
Oxidation of wines due to small volumes and too much tank headspace is probably the most common reason home winemakers experience issues with VA. Popped carboy bungs and deflated variable volume tank lids are also a common oxidation problem for students in my winemaking classes. It is important to periodically monitor wines during aging, especially if they are stored in an area with large temperature fluctuations. Regarding carboys, a properly filled carboy is one where the wine reaches into the narrow neck of the carboy, with only 1–2 inches (2–5 cm) of head space. This is why having a number of different tanks or carboy sizes on hand is useful. Extra wine may be stored in small containers, but in my experience wine stored in 1-gallon (4-L) jugs or smaller will often become oxidized within four to six months. A better option for very small volumes of wine is to bottle them with a tight cork in regular 750 mL bottles.
If wines are bottled correctly, limiting oxygen pickup and with an adequate level of free SO2 at bottling, there is usually very little oxygen ingress during bottle aging and thus limited further large scale oxidation. Still, if wines are close to threshold levels in acetic acid or ethyl acetate, continuing chemical oxidation reactions producing acetaldehyde and equilibrium reactions between the level of acetic acid and ethyl acetate may bring the level of these chemicals above threshold values. Thus it is important to reduce oxidation during every step of post-fermentation production in order to bottle wines with limited oxidation potential prior to consumption.
Commercial wineries often monitor volatile acidity during aging using a special piece of laboratory glassware called a Cash still or a modified Cash still. As the name implies, this apparatus distills volatile acids out of a small wine sample by boiling the sample in a heated water bath. The vapor is then condensed and the slightly acidic distillate is titrated with sodium hydroxide to determine the volatile acid level. While this method is relatively straightforward in terms of execution it does take time, approximately 20–30 minutes per sample, and some practice to titrate accurately using phenolphthalein indicator. Because of the analysis time involved and the cost of the glassware, currently $700–$800, many smaller wineries simply use sensory analysis to determine if specific wine lots have unacceptable levels of volatile acidity.
Unfortunately, if high levels of acetic acid, ethyl acetate, or acetaldehyde have been generated in wines they are practically impossible for the home winemaker to remove. VA aromas cannot be filtered out of wines using traditional filtration methods due to their low molecular weight. Fining is also impractical due to lack of specific binding agents that could preferentially remove these compounds. Fining with activated carbon could be an option, but carbon is very non-specific and most of the positive aroma compounds will probably be removed from the wine before VA aromas are reduced to acceptable levels. In commercial production a combination of near-reverse osmosis filtration and treatment of the filtrate stream with activated carbon is used to remove volatile acid components without stripping all of the other aroma compounds out of the wine. This type of equipment is quite expensive, and hopefully seldom used, so most commercial wineries rely on mobile service companies to treat any wine lots with high levels of VA. For home wine production, prevention is critical to limiting the level of VA in finished wines.
In summary, volatile acidity encompasses several different aroma compounds, all of which are usually considered to negatively impact wine aroma. The aromas of these compounds are often described as nail polish remover or paint thinner, overripe apple, and vinegar. Prevention of unwanted microbial metabolism and limiting oxygen exposure in wines after fermentation are the two key activities necessary to limit production of volatile acid aromas. VA may be monitored using specialized lab equipment, but may also be monitored by using one’s nose. Removing VA aromas is expensive and usually out of reach for home winemakers, therefore prevention is the key to success.
References
1 Waterhouse, A. L. (2016). Acids. In A. L. Waterhouse, Understanding wine chemistry (p. 20). Chichester, West Sussex: John Wiley & Sons, Inc.
2 Waterhouse, A. L. (2016). Aldehydes Ketones and Related Compounds. In A. L. Waterhouse, Understanding wine chemistry (p. 81). Chichester, West Sussex: John Wiley & Sons, Inc.
3 Waterhouse, A. L. (2016). Esters. In A. L. Waterhouse, Understanding wine chemistry (p. 62). Chichester, West Sussex: John Wiley & Sons, Inc.






